Details of the Drug
General Information of Drug (ID: DMWBHRY)
| Drug Name |
Olmesartan medoxomil
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Benevas; Benicar; Olmetec; Olvance; Forest Brand of Olmesartan Medoxomil; Olmesartan medoximil; Sankyo Brand of Olmesartan Medoxomil; Cs 866; Benicar (TN); Berlin-Chemie Brand of Olmesartan Medoxomil; CS-866; CS-866DM; CS-866RN; DE-092; KS-1182; Olmetec (TN); Benicar, Olmetec,Olmesartan; Olmesartan medoxomil (JAN/USAN); (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylate; (5-methyl-2-oxo-1,3-dioxolen-4-yl)methoxy-4-(1-hydroxy-1-methylethyl)-2-propyl-1-(4-(2-(tetrazol-5-yl)phenyl)phenyl)methylimidazol-5-carboxylate; 1H-Imidazole-5-carboxylic acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-((2'-(1H-tetrazol-5-yl) (1,1'-biphenyl)-4-yl)methyl)-, (5-methyl-2-oxo-1,3-dioxol-4-yl) methyl ester; 1H-Imidazole-5-carboxylic acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-((2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester; 1H-imidazole-5-carboxylic acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-((2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-,(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
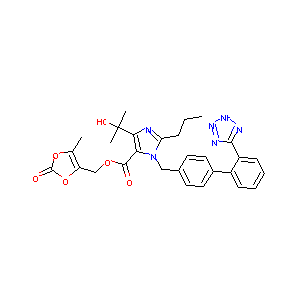 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 558.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | High blood pressure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
