Details of the Drug
General Information of Drug (ID: DMWE60C)
| Drug Name |
Rufinamide
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
106308-44-5; Inovelon; Banzel; 1-(2,6-Difluorobenzyl)-1H-1,2,3-triazole-4-carboxamide; CGP-33101; Cgp 33101; Xilep; 1-[(2,6-difluorophenyl)methyl]-1H-1,2,3-Triazole-4-carboxamide; RUF-331; UNII-WFW942PR79; RUF 331; 1-[(2,6-difluorophenyl)methyl]triazole-4-carboxamide; WFW942PR79; C10H8F2N4O; E 2080; POGQSBRIGCQNEG-UHFFFAOYSA-N; 1H-1,2,3-Triazole-4-carboxamide, 1-[(2,6-difluorophenyl)methyl]-; NCGC00165883-02; E2080; DSSTox_CID_26506; DSSTox_RID_81675; DSSTox_GSID_46506; Banzel; Banzel, Rufinamide; E-2080; SYN-111; Rufinamide (USAN/INN); Inovelon/Banzel
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
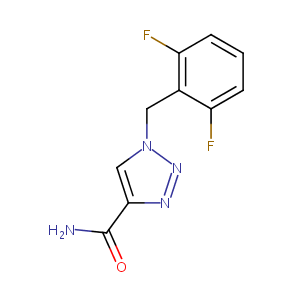 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 238.19 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.7 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Rufinamide
Coadministration of a Drug Treating the Disease Different from Rufinamide (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7470). | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of Eisai. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Emerging drugs for epilepsy. Expert Opin Emerg Drugs. 2007 Sep;12(3):407-22. | ||||
| 8 | Investigation of the metabolism of rufinamide and its interaction with valproate. Drug Metab Lett. 2011 Dec;5(4):280-9. | ||||
| 9 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 10 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 11 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 12 | Altice FL, Friedland GH, Cooney EL "Nevirapine induced opiate withdrawal among injection drug users with HIV infection receiving methadone." AIDS 13 (1999): 957-62. [PMID: 10371177] | ||||
| 13 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 14 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 15 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 16 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 17 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 18 | Product Information. Bosulif (bosutinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 19 | Bruderer S, Aanismaa P, Homery MC, et al. "Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue-targeting dual endothelin receptor antagonist." AAPS J 14 (2012): 68-78. [PMID: 22189899] | ||||
| 20 | Product Information. Hectorol (doxercalciferol). Genzyme Corporation, Cambridge, MA. | ||||
| 21 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 22 | Belcastro V, Costa C, Striano P "Levetiracetam-associated hyponatremia." Seizure 17 (2008): 389-90. [PMID: 18584781] | ||||
| 23 | Product Information. Isturisa (osilodrostat). Recordati Rare Diseases Inc, Lebanon, NJ. | ||||
| 24 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 25 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 26 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 27 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 28 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 29 | Canadian Pharmacists Association. | ||||
| 30 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 31 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 32 | Product Information. Movantik (naloxegol). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 33 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 34 | Product Information. Zykadia (ceritinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 35 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 36 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 37 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 38 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 39 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 40 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 41 | Product Information. Imbruvica (ibrutinib). Pharmacyclics Inc, Sunnyvale, CA. | ||||
| 42 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 43 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 44 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 45 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 46 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 47 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 48 | Product Information. Jakafi (ruxolitinib). Incyte Corporation, Wilmington, DE. | ||||
| 49 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 50 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 51 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 52 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 53 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 54 | Product Information. Xtandi (enzalutamide). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 55 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 56 | Product Information. Adempas (riociguat). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 57 | Product Information. Stendra (avanafil). Vivus Inc, Mountain View, CA. | ||||
| 58 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 59 | Product Information. Nuvigil (armodafinil). Cephalon Inc, West Chester, PA. | ||||
| 60 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 61 | Product Information. Eliquis (apixaban). Bristol-Myers Squibb Canada Inc, Montreal, IN. | ||||
| 62 | Product Information. Cabometyx (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 63 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
