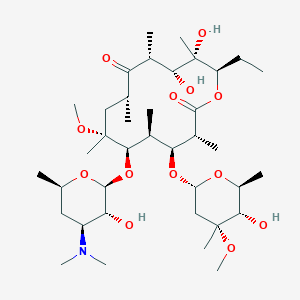
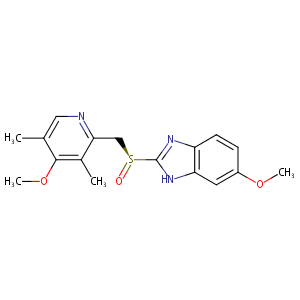
| 1 |
ClinicalTrials.gov (NCT01667575) Efficiency Study of Clarithromycin and Bismuth-containing Quadruple Therapy to Treat H.Pylori
|
| 2 |
Clarithromycin FDA Label
|
| 3 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 4 |
Anti-inflammatory Clarithromycin for Improving COVID-19 Infection Early (ACHIEVE)
|
| 5 |
Esomeprazole FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5488).
|
| 7 |
Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001 Oct 25;413(6858):814-21.
|
| 8 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 9 |
Pharmacokinetic variability of clarithromycin and differences in CYP3A4 activity in patients with cystic fibrosis. J Cyst Fibros. 2014 Mar;13(2):179-85.
|
| 10 |
Drug Interactions Flockhart Table
|
| 11 |
Influence of CYP2C19 polymorphism and Helicobacter pylori genotype determined from gastric tissue samples on response to triple therapy for H pylori infection. Clin Gastroenterol Hepatol. 2005 Jun;3(6):564-73.
|
| 12 |
Use of immortalized human hepatocytes to predict the magnitude of clinical drug-drug interactions caused by CYP3A4 induction. Drug Metab Dispos. 2006 Oct;34(10):1742-8.
|
| 13 |
Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19.
|
| 14 |
A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005 Feb;83(2):282-92.
|
| 15 |
Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43.
|
| 16 |
A genome-wide analysis of targets of macrolide antibiotics in mammalian cells. J Biol Chem. 2020 Feb 14;295(7):2057-2067. doi: 10.1074/jbc.RA119.010770. Epub 2020 Jan 8.
|
| 17 |
Liver toxicity of macrolide antibiotics in zebrafish. Toxicology. 2020 Aug;441:152501. doi: 10.1016/j.tox.2020.152501. Epub 2020 May 23.
|
| 18 |
Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother. 2005 Sep;56(3):502-6. doi: 10.1093/jac/dki251. Epub 2005 Jul 8.
|
| 19 |
T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001 Jun;107(11):1433-41. doi: 10.1172/JCI12118.
|
| 20 |
Effects of eight antibacterial agents on cell survival and expression of epithelial-cell- or cell-adhesion-related genes in human gingival epithelial cells. J Periodontal Res. 2004 Feb;39(1):50-8. doi: 10.1111/j.1600-0765.2004.00704.x.
|
| 21 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 22 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 23 |
Predictive performance of physiologically based pharmacokinetic (PBPK) modeling of drugs extensively metabolized by major cytochrome P450s in children. Clin Pharmacol Ther. 2018 Jul;104(1):188-200.
|
| 24 |
The effects of drugs with immunosuppressive or immunomodulatory activities on xenobiotics-metabolizing enzymes expression in primary human hepatocytes. Toxicol In Vitro. 2015 Aug;29(5):1088-99.
|
| 25 |
ClinicalTrials.gov (NCT04090021) Genotypic Resistance-guided Versus Empirical Therapy for H. Pylori Eradication.
|
| 26 |
ClinicalTrials.gov (NCT02296021) Helicobacter Pylori Eradication With Berberine Quadruple Therapy Versus Bismuth-containing Quadruple Therapy
|
| 27 |
ClinicalTrials.gov (NCT02732249) Clarithromycin Triple Therapy Plus Bismuth for Helicobacter Pylori First-line Treatment
|
| 28 |
ClinicalTrials.gov (NCT02552641) Food Effect on the Eradication Rate of H. Pylori With Triple Therapy With Esomeprazole
|
| 29 |
ClinicalTrials.gov (NCT06162949) Vonoprazan for Helicobacter Pylori Eradication in Adolescents
|
| 30 |
ClinicalTrials.gov (NCT03383003) Comparison of Two Novel First-line Anti-Helicobacter Pylori Therapy
|
| 31 |
ClinicalTrials.gov (NCT05189444) The Evaluation of Triple Therapy With Vonoprazan, Amoxicillin and Bismuth for Eradication of Helicobacter Pylori
|
| 32 |
ClinicalTrials.gov (NCT04753437) A Study of Vonoprazan in Adults With Helicobacter Pylori
|
|
|
|
|
|
|