| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
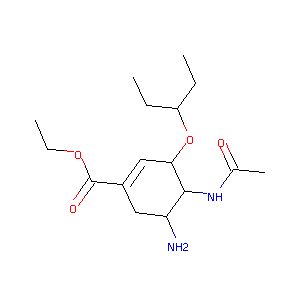
Oseltamivir FDA Label
|
| 3 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 4 |
ClinicalTrials.gov (NCT04261270) A Randomized,Open,Controlled Clinical Study to Evaluate the Efficacy of ASC09F and Ritonavir for 2019-nCoV Pneumonia
|
| 5 |
Atenolol FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 548).
|
| 7 |
Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102.
|
| 8 |
Nonclinical pharmacokinetics of oseltamivir and oseltamivir carboxylate in the central nervous system. Antimicrob Agents Chemother. 2009 Nov;53(11):4753-61.
|
| 9 |
Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos. 2009 Feb;37(2):315-21.
|
| 10 |
Oseltamivir (tamiflu) is a substrate of peptide transporter 1. Drug Metab Dispos. 2009 Aug;37(8):1676-81.
|
| 11 |
FDA Drug Development and Drug Interactions
|
| 12 |
An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20.
|
| 13 |
In vitro inhibition of carboxylesterase 1 by Kava (Piper methysticum) Kavalactones. Chem Biol Interact. 2022 Apr 25;357:109883. doi: 10.1016/j.cbi.2022.109883. Epub 2022 Mar 9.
|
| 14 |
Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol. 2007 Sep;72(3):686-94. doi: 10.1124/mol.107.036889. Epub 2007 May 30.
|
| 15 |
Change in mRNA Expression after Atenolol, a Beta-adrenergic Receptor Antagonist and Association with Pharmacological Response. Arch Drug Inf. 2009 Sep;2(3):41-50. doi: 10.1111/j.1753-5174.2009.00020.x.
|
| 16 |
Prediction and experimental validation of acute toxicity of beta-blockers in Ceriodaphnia dubia. Environ Toxicol Chem. 2005 Oct;24(10):2470-6.
|
| 17 |
Interaction of digoxin with antihypertensive drugs via MDR1. Life Sci. 2002 Feb 15;70(13):1491-500.
|
| 18 |
Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42.
|
| 19 |
Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010 Nov;70(5):645-55.
|
| 20 |
Metabolism of atenolol in man. Xenobiotica. 1978 May;8(5):313-20.
|
| 21 |
Application of substrate depletion assay to evaluation of CYP isoforms responsible for stereoselective metabolism of carvedilol. Drug Metab Pharmacokinet. 2016 Dec;31(6):425-432.
|
| 22 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 23 |
Additional gene variants reduce effectiveness of beta-blockers in the LQT1 form of long QT syndrome. J Cardiovasc Electrophysiol. 2004 Feb;15(2):190-9. doi: 10.1046/j.1540-8167.2004.03212.x.
|
| 24 |
Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43.
|
| 25 |
Effects of the direct renin inhibitor aliskiren and atenolol alone or in combination in patients with hypertension. J Renin Angiotensin Aldosterone Syst. 2008 Sep;9(3):163-75. doi: 10.1177/1470320308096411.
|
| 26 |
Long-term effects of irbesartan and atenolol on the renin-angiotensin-aldosterone system in human primary hypertension: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). J Cardiovasc Pharmacol. 2003 Dec;42(6):719-26. doi: 10.1097/00005344-200312000-00005.
|
| 27 |
Atenolol improves ventricular function without changing plasma noradrenaline but decreasing plasma atrial natriuretic factor in chronic heart failure. Auton Autacoid Pharmacol. 2002 Oct-Dec;22(5-6):261-8. doi: 10.1046/j.1474-8673.2002.00266.x.
|
| 28 |
The effects of clonidine hydrochloride versus atenolol monotherapy on serum lipids, lipid subfractions, and apolipoproteins in mild hypertension. Am Heart J. 1990 Jul;120(1):172-9. doi: 10.1016/0002-8703(90)90175-w.
|
| 29 |
Atenolol-induced regulation of leukocyte beta 2-adrenoceptors in hypertension. Pharmacology. 1984;29(4):210-4. doi: 10.1159/000138015.
|
| 30 |
A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008 Feb;26(2):351-6. doi: 10.1097/HJH.0b013e3282f283c9.
|
| 31 |
Folic acid uptake by the human syncytiotrophoblast: interference by pharmacotherapy, drugs of abuse and pathological conditions. Reprod Toxicol. 2009 Dec;28(4):511-20. doi: 10.1016/j.reprotox.2009.07.001. Epub 2009 Jul 16.
|
| 32 |
A 1-year follow-up study on the effect of atenolol on serum beta 2-microglobulin level in hypertensive diabetic patients. J Int Med Res. 1989 Mar-Apr;17(2):162-7. doi: 10.1177/030006058901700208.
|
| 33 |
Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003 May 20;107(19):2459-66. doi: 10.1161/01.CIR.0000068316.53218.49. Epub 2003 May 12.
|
| 34 |
CACNA1C gene polymorphisms, cardiovascular disease outcomes, and treatment response. Circ Cardiovasc Genet. 2009 Aug;2(4):362-70. doi: 10.1161/CIRCGENETICS.109.857839. Epub 2009 Jun 3.
|
| 35 |
B2 bradykinin receptor (B2BKR) polymorphism and change in left ventricular mass in response to antihypertensive treatment: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) trial. J Hypertens. 2003 Mar;21(3):621-4. doi: 10.1097/00004872-200303000-00029.
|
| 36 |
Gender-specific association between preproendothelin-1 genotype and reduction of systolic blood pressure during antihypertensive treatment--results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). Clin Cardiol. 2004 May;27(5):287-90. doi: 10.1002/clc.4960270510.
|
| 37 |
Single nucleotide polymorphisms predict the change in left ventricular mass in response to antihypertensive treatment. J Hypertens. 2004 Dec;22(12):2321-8. doi: 10.1097/00004872-200412000-00014.
|
|
|
|
|
|
|