Details of the Drug
General Information of Drug (ID: DMGO72P)
| Drug Name |
Oseltamivir
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Tamiflu (*Phosphate salt 1:1*); Agucort; Oseltamivirum; Tamvir; GS 4104; GS4104; Agucort (TN); GS-4104; Oseltamivir (INN); Oseltamivir [INN:BAN]; Ro-640796; Tamiflu (TN); Tamiflu-Free; Ro-64-0796; OTV | ||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Influenza A virusInfluenza B virus
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
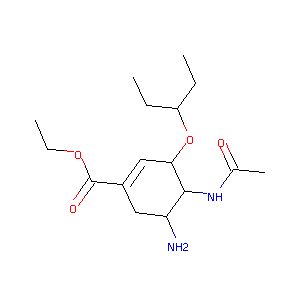 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 312.4 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.1 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Oseltamivir FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
| 3 | ClinicalTrials.gov (NCT04261270) A Randomized,Open,Controlled Clinical Study to Evaluate the Efficacy of ASC09F and Ritonavir for 2019-nCoV Pneumonia | ||||
| 4 | Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102. | ||||
| 5 | Oseltamivir (tamiflu) is a substrate of peptide transporter 1. Drug Metab Dispos. 2009 Aug;37(8):1676-81. | ||||
| 6 | Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos. 2009 Feb;37(2):315-21. | ||||
| 7 | FDA Drug Development and Drug Interactions | ||||
| 8 | Nonclinical pharmacokinetics of oseltamivir and oseltamivir carboxylate in the central nervous system. Antimicrob Agents Chemother. 2009 Nov;53(11):4753-61. | ||||
| 9 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 10 | Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol. 2007 Sep;72(3):686-94. doi: 10.1124/mol.107.036889. Epub 2007 May 30. | ||||
| 11 | In vitro inhibition of carboxylesterase 1 by Kava (Piper methysticum) Kavalactones. Chem Biol Interact. 2022 Apr 25;357:109883. doi: 10.1016/j.cbi.2022.109883. Epub 2022 Mar 9. | ||||
