| 1 |
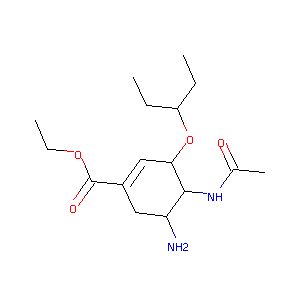
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Oseltamivir FDA Label
|
| 3 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 4 |
ClinicalTrials.gov (NCT04261270) A Randomized,Open,Controlled Clinical Study to Evaluate the Efficacy of ASC09F and Ritonavir for 2019-nCoV Pneumonia
|
| 5 |
Effect of angiotensin receptor blockade on endothelial function: focus on olmesartan medoxomil. Vasc Health Risk Manag. 2009;5(1):301-14.
|
| 6 |
Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102.
|
| 7 |
Nonclinical pharmacokinetics of oseltamivir and oseltamivir carboxylate in the central nervous system. Antimicrob Agents Chemother. 2009 Nov;53(11):4753-61.
|
| 8 |
Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos. 2009 Feb;37(2):315-21.
|
| 9 |
Oseltamivir (tamiflu) is a substrate of peptide transporter 1. Drug Metab Dispos. 2009 Aug;37(8):1676-81.
|
| 10 |
FDA Drug Development and Drug Interactions
|
| 11 |
An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20.
|
| 12 |
In vitro inhibition of carboxylesterase 1 by Kava (Piper methysticum) Kavalactones. Chem Biol Interact. 2022 Apr 25;357:109883. doi: 10.1016/j.cbi.2022.109883. Epub 2022 Mar 9.
|
| 13 |
Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol. 2007 Sep;72(3):686-94. doi: 10.1124/mol.107.036889. Epub 2007 May 30.
|
| 14 |
Mechanism of diastolic stiffening of the failing myocardium and its prevention by angiotensin receptor and calcium channel blockers. J Cardiovasc Pharmacol. 2009 Jul;54(1):47-56.
|
| 15 |
Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007 Dec;35(12):2166-76.
|
| 16 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
|
| 17 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01913)
|
| 18 |
OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab Dispos. 2006 May;34(5):862-9.
|
| 19 |
A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci. 2013 Nov;136(1):216-41.
|
| 20 |
Immunopathogenesis of olmesartan-associated enteropathy. Aliment Pharmacol Ther. 2015 Dec;42(11-12):1303-14. doi: 10.1111/apt.13413. Epub 2015 Oct 1.
|
|
|
|
|
|
|