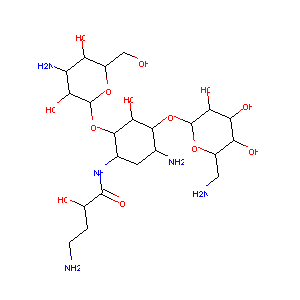
| 1 |
ClinicalTrials.gov (NCT01867138) Neonatal Suspected Sepsis Treated With Cefazolin or Vancomycin
|
| 2 |
Amikacin FDA Label
|
| 3 |
Emerging drugs for bacterial urinary tract infections. Expert Opin Emerg Drugs. 2005 May;10(2):275-98.
|
| 4 |
The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954.
|
| 5 |
Cefazolin FDA Label
|
| 6 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 065244.
|
| 7 |
Modulation of melanogenesis and antioxidant defense system in melanocytes by amikacin. Toxicol In Vitro. 2013 Apr;27(3):1102-8.
|
| 8 |
Bacterial resistance to aminoglycosides and beta-lactams: the Tn1331 transposon paradigm. Front Biosci. 2000 Jan 1;5:D20-9.
|
| 9 |
Relationship between antimicrobial resistance and aminoglycoside-modifying enzyme gene expressions in Acinetobacter baumannii. Chin Med J (Engl). 2005 Jan 20;118(2):141-5.
|
| 10 |
Expression of Clostridium thermocellum endoglucanase gene in Lactobacillus gasseri and Lactobacillus johnsonii and characterization of the genetically modified probiotic lactobacilli. Curr Microbiol. 2000 Apr;40(4):257-63.
|
| 11 |
An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20.
|
| 12 |
Amikacin-induced type 5 Bartter-like syndrome with severe hypocalcemia. J Postgrad Med. 2009 Jul-Sep;55(3):208-10. doi: 10.4103/0022-3859.57407.
|
| 13 |
Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Hum Mol Genet. 2009 Apr 1;18(7):1310-22. doi: 10.1093/hmg/ddp030. Epub 2009 Jan 15.
|
| 14 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 15 |
Bacteriological characteristics of Staphylococcus aureus isolates from humans and bulk milk. J Dairy Sci. 2008 Feb;91(2):564-9.
|
| 16 |
Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157-81.
|
| 17 |
Oral availability of cefadroxil depends on ABCC3 and ABCC4. Drug Metab Dispos. 2012 Mar;40(3):515-21.
|
| 18 |
Three-dimensional quantitative structure-activity relationship analyses of beta-lactam antibiotics and tripeptides as substrates of the mammalian H+/peptide cotransporter PEPT1. J Med Chem. 2005 Jun 30;48(13):4410-9.
|
| 19 |
Expression levels of renal organic anion transporters (OATs) and their correlation with anionic drug excretion in patients with renal diseases. Pharm Res. 2004 Jan;21(1):61-7.
|
| 20 |
Cefazolin administration and 2-methyl-1,3,4-thiadiazole-5-thiol in human tissue: possible relationship to hypoprothrombinemia. Drug Metab Dispos. 2002 Oct;30(10):1123-8.
|
| 21 |
Analysis of the drug-resistant characteristics of Klebsiella pneumoniae isolated from the respiratory tract and CTX-M ESBL genes. Genet Mol Res. 2015 Oct 5;14(4):12043-8.
|
| 22 |
Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J Microbiol Immunol Infect. 2018 Aug;51(4):565-572.
|
| 23 |
Prevalence of antimicrobial resistance genes in Bacteroides spp. and Prevotella spp. Dutch clinical isolates. Clin Microbiol Infect. 2019 Sep;25(9):1156.e9-1156.e13.
|
|
|
|
|
|
|