Details of the Drug
General Information of Drug (ID: DM5PDRB)
| Drug Name |
Amikacin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Amicacin; Amikacina; Amikacine; Amikacinum; Amikavet; Amikin; Arikace; Briclin; Kaminax; Lukadin; Mikavir; AMIKACIN SULFATE; Amikacin Base; Amikacin Dihydrate; ANTIBIOTIC BB-K8; Amiglyde-V; Amikacin & Tumor Necrosis Factor; Amikacin (USP); Amikacina [INN-Spanish]; Amikacine [INN-French]; Amikacinum [INN-Latin]; Amikin(Disulfate); Antibiotic BB-K 8; BB-K 8; BB-K8; Amiglyde-V (TN); Amikacin (USP/INN); Amikacin [USAN:BAN:INN]; O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(6-amino-6-deoxy-alpha-D-glucopyranosyl-(1-6))-N(sup 3)-(4-amino-L-2-hydroxybutyryl)-2-deoxy-L-streptamine; O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1->4)-O-(6-amino-6-deoxy-alpha-D-glucopyranosyl-(1->6))-N(3)-(4-amino-L-2-hydroxybutyryl)-2-deoxy-L-streptamine; O-3-Amino-3-deoxy-.alpha.-D-glucopyranosyl-(1->6)-O-[6-amino-6-deoxy-.alpha.-D-glucopyranosyl-(1->4)]-1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-D-streptamine; (2S)-4-amino-N-[(1R,2S,3S,4R,5S)-5-amino-2-(3-amino-3-deoxy-alpha-D-glucopyranosyloxy)-4-(6-amino-6-deoxy-alpha-D-glucopyranosyloxy)-3-hydroxycyclohexyl]-2-hydroxybutanamide; (2S)-4-amino-N-[(1R,2S,3S,4R,5S)-5-amino-2-[(2S,3R,4S,5S,6R)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-[(2R,3R,4S,5S,6R)-6-(aminomethyl)-3,4,5-trihydroxyoxan-2-yl]oxy-3-hydroxycyclohexyl]-2-hydroxybutanamide; (2S)-4-amino-N-{(1R,2S,3S,4R,5S)-5-amino-2-[(3-amino-3-deoxy-alpha-D-glucopyranosyl)oxy]-4-[(6-amino-6-deoxy-alpha-D-glucopyranosyl)oxy]-3-hydroxycyclohexyl}-2-hydroxybutanamide; 1-N-(L(-)-gamma-Amino-alpha-hydroxybutyryl)kanamycin A
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteriaHumans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
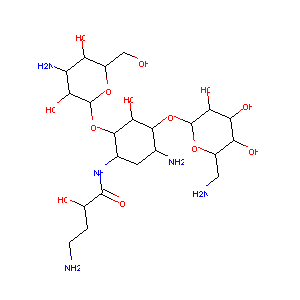 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 585.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -7.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 17 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Amikacin
Coadministration of a Drug Treating the Disease Different from Amikacin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Amikacin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Emerging drugs for bacterial urinary tract infections. Expert Opin Emerg Drugs. 2005 May;10(2):275-98. | ||||
| 3 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Bacterial resistance to aminoglycosides and beta-lactams: the Tn1331 transposon paradigm. Front Biosci. 2000 Jan 1;5:D20-9. | ||||
| 9 | Relationship between antimicrobial resistance and aminoglycoside-modifying enzyme gene expressions in Acinetobacter baumannii. Chin Med J (Engl). 2005 Jan 20;118(2):141-5. | ||||
| 10 | Expression of Clostridium thermocellum endoglucanase gene in Lactobacillus gasseri and Lactobacillus johnsonii and characterization of the genetically modified probiotic lactobacilli. Curr Microbiol. 2000 Apr;40(4):257-63. | ||||
| 11 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 12 | Modulation of melanogenesis and antioxidant defense system in melanocytes by amikacin. Toxicol In Vitro. 2013 Apr;27(3):1102-8. | ||||
| 13 | Amikacin-induced type 5 Bartter-like syndrome with severe hypocalcemia. J Postgrad Med. 2009 Jul-Sep;55(3):208-10. doi: 10.4103/0022-3859.57407. | ||||
| 14 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | FDA. U.S. Food and Drug Administration "FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs).". | ||||
| 17 | Engle JE, Drago J, Carlin B, Schoolwerth AC "Letter: Reversible acute renal failure after cephalothin." Ann Intern Med 83 (1975): 232-3. [PMID: 1147461] | ||||
| 18 | Piper BJ, Alinea AA, Wroblewski JR, et al. A Quantitative and Narrative Evaluation of Goodman and Gilman's Pharmacological Basis of Therapeutics. Pharmacy (Basel). 2019;8(1):1. Published 2019 Dec 20. [PMID: 31861770] | ||||
| 19 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 20 | Chang JT, Green L, Beitz J "Renal failure with the use of zoledronic acid." N Engl J Med 349 (2003): 1676-9 discussion 1676-9. [PMID: 14573746] | ||||
| 21 | Athlin L, Domellof L, Holm S "Gentamicin treatment in severe surgical infections: serum levels, interactions, toxicity and efficacy." Acta Chir Scand 147 (1981): 225-30. [PMID: 7034430] | ||||
| 22 | Wong GT, Lee EY, Irwin MG. Contrast induced nephropathy in vascular surgery.?Br J Anaesth. 2016;117 Suppl 2:ii63-ii73. [PMID: 27566809] | ||||
| 23 | Assael BM, Chiabrando C, Gagliardi L, Noseda A, Bamonte F, Salmona M "Prostaglandins and aminoglycoside nephrotoxicity." Toxicol Appl Pharmacol 78 (1985): 386-94. [PMID: 4049389] | ||||
| 24 | Product Information. Eloxatin (oxaliplatin). Sanofi Winthrop Pharmaceuticals, New York, NY. | ||||
| 25 | Burkett L, Bikhazi GB, Thomas KC Jr, Rosenthal DA, Wirta MG, Foldes FF "Mutual potentiation of the neuromuscular effects of antibiotics and relaxants." Anesth Analg 58 (1979): 107-15. [PMID: 571233] | ||||
| 26 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 27 | Bates DE, Beaumont SJ, Baylis BW "Ototoxicity induced by gentamicin and furosemide." Ann Pharmacother 36 (2002): 446-51. [PMID: 11895059] | ||||
| 28 | Churchill DN, Seely J "Nephrotoxicity associated with combined gentamicin - amphotericin B therapy." Nephron 19 (1977): 176-81. [PMID: 268496] | ||||
| 29 | Novis BH, Korzets Z, Chen P, Bernheim J "Nephrotic syndrome after treatment with 5-aminosalicylic acid." Br Med J (Clin Res Ed) 296 (1988): 1442. [PMID: 3132281] | ||||
| 30 | Farag MM, Mikhail MR, Abdel-Meguid E, Abdel-Tawab S "Assessment of gentamicin-induced nephrotoxicity in rats treated with low doses of ibuprofen and diclofenac sodium." Clin Sci 91 (1996): 187-91. [PMID: 8795442] | ||||
| 31 | Banerjee D, Asif A, Striker L, Preston RA, Bourgoignie JJ, Roth D "Short-term, high-dose pamidronate-induced acute tubular necrosis: The postulated mechanisms of bisphosphonate nephrotoxicity." Am J Kidney Dis 41 (2003): E18. [PMID: 12778436] | ||||
| 32 | Product Information. Clolar (clofarabine). sanofi-aventis, Bridgewater, NJ. | ||||
| 33 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Paraplatin (carboplatin). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 35 | Product Information. Nexium (esomeprazole) Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 36 | Product Information. Alimta (pemetrexed). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 37 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
