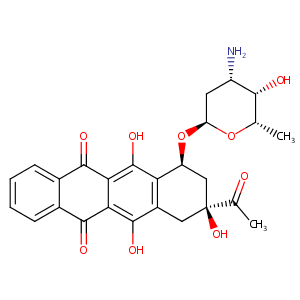
| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
Idarubicin FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7083).
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7945).
|
| 5 |
UBE2T promotes nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by activating the AKT/GSK3/-catenin pathway.Oncotarget. 2016 Mar 22;7(12):15161-72.
|
| 6 |
Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Sci Rep. 2014 Sep 26;4:6437. doi: 10.1038/srep06437.
|
| 7 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 8 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 9 |
Amonafide L-malate is not a substrate for multidrug resistance proteins in secondary acute myeloid leukemia. Leukemia. 2008 Nov;22(11):2110-5.
|
| 10 |
In vitro evaluation of cytochrome P450-mediated drug interactions between cytarabine, idarubicin, itraconazole and caspofungin. Hematology. 2004 Jun;9(3):217-21.
|
| 11 |
A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro. J Am Soc Nephrol. 2016 Apr;27(4):1015-28. doi: 10.1681/ASN.2015010060. Epub 2015 Aug 10.
|
| 12 |
The use of biochemical markers in cardiotoxicity monitoring in patients treated for leukemia. Neoplasma. 2005;52(5):430-4.
|
| 13 |
The induction of apoptosis by daunorubicin and idarubicin in human trisomic and diabetic fibroblasts. Cell Mol Biol Lett. 2008;13(2):182-94. doi: 10.2478/s11658-007-0045-7. Epub 2008 Apr 10.
|
| 14 |
Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19.
|
| 15 |
Chemerin promotes the pathogenesis of preeclampsia by activating CMKLR1/p-Akt/CEBP axis and inducing M1 macrophage polarization. Cell Biol Toxicol. 2022 Aug;38(4):611-628. doi: 10.1007/s10565-021-09636-7. Epub 2021 Aug 16.
|
| 16 |
First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors.J Clin Oncol.2011 Dec 10;29(35):4688-95.
|
| 17 |
Platycodin D potentiates proliferation inhibition and apoptosis induction upon AKT inhibition via feedback blockade in non-small cell lung cancer cells. Sci Rep. 2016 Nov 29;6:37997. doi: 10.1038/srep37997.
|
| 18 |
Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects. Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008.
|
| 19 |
PI3K/AKT inhibitors aggravate death receptor-mediated hepatocyte apoptosis and liver injury. Toxicol Appl Pharmacol. 2019 Oct 15;381:114729. doi: 10.1016/j.taap.2019.114729. Epub 2019 Aug 22.
|
| 20 |
Harnessing the PI3K/Akt/mTOR pathway in T-cell acute lymphoblastic leukemia: eliminating activity by targeting at different levels. Oncotarget. 2012 Aug;3(8):811-23. doi: 10.18632/oncotarget.579.
|
| 21 |
-Mangostin alleviates liver fibrosis through Sirtuin 3-superoxide-high mobility group box 1 signaling axis. Toxicol Appl Pharmacol. 2019 Jan 15;363:142-153. doi: 10.1016/j.taap.2018.11.011. Epub 2018 Nov 29.
|
| 22 |
Akt activation by Ca(2+)/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J Biol Chem. 2017 Aug 25;292(34):14188-14204. doi: 10.1074/jbc.M117.778464. Epub 2017 Jun 20.
|
|
|
|
|
|
|