Details of the Drug
General Information of Drug (ID: DM3VOGS)
| Drug Name |
Ceftizoxime
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ceftix; Ceftizoxima; Ceftizoximum; Eposerin; Ceftizoxime Monosodium Salt; FK749; FR 13749; Cefizox (TN); Ceftizoxima[INN-Spanish]; Ceftizoxime (INN); Ceftizoxime [INN:BAN]; Ceftizoximum [INN-Latin]; FK-749; FR-13479; FR-13749; SKF-88373; SK&F 88373-2; Syn-7-(2-(2-Amino-4-thiazolyl)-2-methoxyiminoacetamido)-3-cephem-4-carboxylic acid; (6R,7R)-7-(2-(2-Amino-4-thiazolyl)-2Z-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure; (6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxyamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-carbonsaeure-7-(Z)-(O-methyloxim); (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7beta-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-2,3-didehydropenam-2-carboxylic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Staphylococcus aureusStaphylococcus epidermidisStreptococcus agalactiaeStreptococcus pneumoniaeStreptococcus pyogenesEnterobacterEscherichia coliHaemophilus influenzaeKlebsiella pneumoniaeMorganella morganiiNeisseria gonorrhoeaeProteus mirabilisProteus vulgarisProvidencia rettgeriPseudomonas aeruginosaSerratia marcescensBacteroidesPeptococcus spp.PeptostreptococcusAeromonas hydrophilaCitrobacter spp.Moraxella catarrhalisNeisseria meningitidisProvidencia stuartii
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
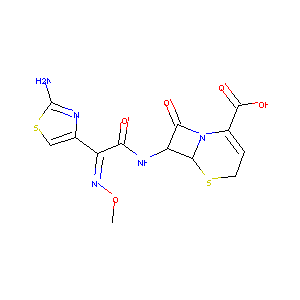 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 383.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ceftizoxime (Comorbidity)
|
|||||||||||||||||||||||||||||
References
