Details of the Drug
General Information of Drug (ID: DM4LSNE)
| Drug Name |
Procaine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Allocaine; Anticort; Anuject; Duracaine; Gerokit; Gerovital; Jenacain; Jenacaine; Nissocaine; Norocaine; Novocain; Novocaine; Procain; Procaina; Procainum; Scurocaine; Spinocaine; Solution of novocain; Factor H3; SP01; SP01A; Stoff H3; Vitamin H3; Anticort (TM); Diethylaminoethyl p-aminobenzoate; Gerovital H-3; Novocain (TN); P-Aminobenzoyldiethylaminoethanol; P-Aminobenzyoyldiethylaminoethanol; Procaina [INN-Spanish]; Procaine (INN); Procaine [INN:BAN]; Procaine, base; Procainum [INN-Latin]; SP-01A; Solution of novocain (TN); Beta-Diethylaminoethyl 4-aminobenzoate; P-Aminobenzoic acid 2-diethylaminoethyl ester; Beta-(Diethylamino)ethyl 4-aminobenzoate; Beta-(Diethylamino)ethyl p-aminobenzoate; BENZOIC ACID,4-AMINO,2-DIETHYLAMINOETHYL ESTER PROCAIN BASE; Benzoic acid, 4-amino-, 2-(diethylamino)ethyl ester; Benzoic acid, p-amino-, 2-(diethylamino)ethyl ester; 2-(Diethylamino)ethyl 4-aminobenzoate; 2-(Diethylamino)ethyl p-aminobenzoate; 2-(Diethylamino)ethyl-4-aminobenzoate; 2-Diethylaminoethyl 4-aminobenzoate; 2-Diethylaminoethyl p-aminobenzoate; 2-Diethylaminoethylester kyseliny p-aminobenzoove; 2-Diethylaminoethylester kyseliny p-aminobenzoove [Czech]; 4-Aminobenzoesaeure-beta-diethylaminoethylester; 4-Aminobenzoic acid 2-(diethylamino) ethyl ester; 4-Aminobenzoic acid diethylaminoethyl ester
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
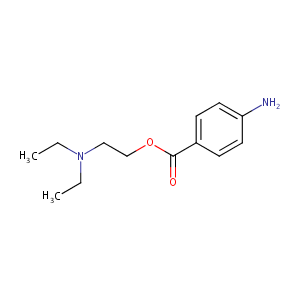 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 236.31 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Anaesthesia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 9A78.6 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Procaine
Coadministration of a Drug Treating the Disease Different from Procaine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 4 | Local anesthetics have different mechanisms and sites of action at recombinant 5-HT3 receptors. Reg Anesth Pain Med. 2007 Nov-Dec;32(6):462-70. | ||||
| 5 | H(1)R mediates local anesthetic-induced vascular permeability in angioedema. Toxicol Appl Pharmacol. 2020 Apr 1;392:114921. doi: 10.1016/j.taap.2020.114921. Epub 2020 Feb 12. | ||||
| 6 | Sensitivity of human dental pulp cells to eighteen chemical agents used for endodontic treatments in dentistry. Odontology. 2013 Jan;101(1):43-51. | ||||
| 7 | Determination and analysis of single nucleotide polymorphisms and haplotype structure of the human carboxylesterase 2 gene. Pharmacogenetics. 2004 Sep;14(9):595-605. doi: 10.1097/00008571-200409000-00004. | ||||
| 8 | Screening of a chemical library reveals novel PXR-activating pharmacologic compounds. Toxicol Lett. 2015 Jan 5;232(1):193-202. doi: 10.1016/j.toxlet.2014.10.009. Epub 2014 Oct 16. | ||||
| 9 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 10 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
