Details of the Drug
General Information of Drug (ID: DM4S3O8)
| Drug Name |
Ertapenem
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ertapenem [INN]; Ertapenem (INN); Invanz (TN); (1R,5S,6S,8R,2'S,4'S)-2-(2-(3-carboxyphenylcarbamoyl)pyrrolidin-4-ylthio)-6-(1-hydroxyethyl)-1-methylcarbapenem-3-carboxylic acid; (4R,5S,6S)-3-((3S,5S)-5-((3-carboxyphenyl)carbamoyl)pyrrolidin-3-ylthio)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (4R,5S,6S)-3-({(3S,5S)-5-[(3-carboxyphenyl)carbamoyl]pyrrolidin-3-yl}sulfanyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (4R,5S,6S)-3-[(3S,5S)-5-[(3-carboxyphenyl)carbamoyl]pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteriaStaphylococcus aureusStreptococcus agalactiaeStreptococcus pneumoniaeStreptococcus pyogenesEscherichia coliHaemophilus influenzaeKlebsiella pneumoniaeMoraxella catarrhalisProteus mirabilisBacteroides fragilisParabacteroides distasonisBacteroides ovatusBacteroides thetaiotaomicronBacteroides uniformisClostridium clostridioformeEubacterium spp.PeptostreptococcusPrevotella biviaPorphyromonas asaccharolytica
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
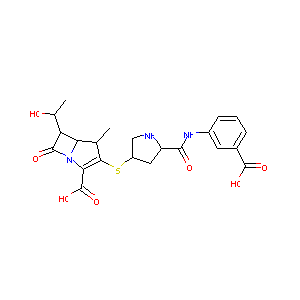 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 475.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.5 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ertapenem (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
