Details of the Drug
General Information of Drug (ID: DM52CPU)
| Drug Name |
ISOCLOZAPINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ISOCLOZAPINE; BRN 0763655; CHEMBL415300; 1977-08-8; 2-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo(b,e)(1,4)diazepine; 2-Chloro-11-(4-methyl-1-piperazinyl)-5H-benzo(b,e)(1,4)diazepine; 5H-DIBENZO(b,e)(1,4)DIAZEPINE, 2-CHLORO-11-(4-METHYL-1-PIPERAZINYL)-; SCHEMBL140207; AC1L279R; CTK4E2258; DTXSID40173464; MolPort-042-665-648; ZINC25973757; BDBM50010594; 2-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine; NCGC00386501-01; LS-60880; 5H-Dibenzo[b,e][1,4]diazepine,2-chloro-11-(4-methyl-1-piperazinyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
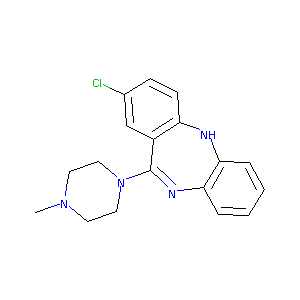 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 326.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
