Details of the Drug
General Information of Drug (ID: DMCYVDT)
| Drug Name |
Chlorzoxazone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Biomioran; CLW; Chloroxazone; Chlorsoxazone; Chlorzoxane; Chlorzoxazon; Chlorzoxazona; Chlorzoxazonum; Clorzoxazona; Escoflex; Klorzoxazon; Mioran; Miotran; Myoflexin; Myoflexine; Neoflex; Nyoflex; Paraflex; Parafon; Pathorysin; Relaxazone; Remofleks; Remular; Solaxin; Component of Parafon Forte; McNeil Brand of Chlorzoxazone; Ortho Brand of Chlorzoxazone; Parafon Forte; Parafon Forte DSC; Strifon Forte Dsc; C 4397; Chlorzoxazonum [INN-Latin]; Clorzoxazona [INN-Spanish]; EZE-DS; Muscol (TN); Paraflex (TN); Parafon Forte (TN); Remular-S; Usaf ma-10; Chlorzoxazone [INN:BAN:JAN]; Chlorzoxazone (JAN/USP/INN); 2-Hydroxy-5-chlorobenzoxazole; 5-Chlorbenzoxazolin-2-on; 5-Chloro-1,3-benzoxazol-2(3H)-one; 5-Chloro-2(3H)-benzoxazolone; 5-Chloro-2-benzoxazolinone; 5-Chloro-2-benzoxazolol; 5-Chloro-2-benzoxazolone; 5-Chloro-2-hydroxybenzoxazole; 5-Chloro-3(H)-2-benzoxazolone; 5-Chlorobenzoksazolinon-2; 5-Chlorobenzoksazolinon-2 [Polish]; 5-Chlorobenzoksazolon-2; 5-Chlorobenzoksazolon-2 [Polish]; 5-Chlorobenzoxazol-2-one; 5-Chlorobenzoxazolidone; 5-Chlorobenzoxazolone; 5-chloro-1,3-benzoxazol-2-ol; 5-chloro-3H-1,3-benzoxazol-2-one; 5-chlorobenzoxazolin-2-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
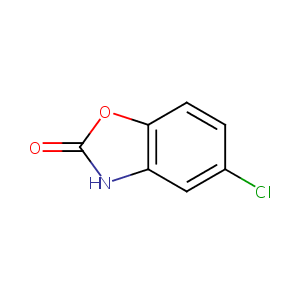 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 169.56 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Chlorzoxazone
Coadministration of a Drug Treating the Disease Different from Chlorzoxazone (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2322). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 4 | Chlorzoxazone inhibits contraction of rat thoracic aorta. Eur J Pharmacol. 2006 Sep 18;545(2-3):161-6. | ||||
| 5 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 6 | Trimethadione metabolism by human liver cytochrome P450: evidence for the involvement of CYP2E1. Xenobiotica. 1998 Nov;28(11):1041-7. | ||||
| 7 | Prediction of human liver microsomal oxidations of 7-ethoxycoumarin and chlorzoxazone with kinetic parameters of recombinant cytochrome P-450 enzymes. Drug Metab Dispos. 1999 Nov;27(11):1274-80. | ||||
| 8 | Inhibitory monoclonal antibodies to human cytochrome P450 1A2: analysis of phenacetin O-deethylation in human liver. Pharmacogenetics. 1998 Oct;8(5):375-82. | ||||
| 9 | Wild-type CYP102A1 as a biocatalyst: turnover of drugs usually metabolised by human liver enzymes. J Biol Inorg Chem. 2007 Mar;12(3):313-23. | ||||
| 10 | The bacterial P450 BM3: a prototype for a biocatalyst with human P450 activities. Trends Biotechnol. 2007 Jul;25(7):289-98. | ||||
| 11 | Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin Pharmacol Ther. 2003 Nov;74(5):468-74. doi: 10.1016/j.clpt.2003.07.001. | ||||
| 12 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 13 | Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004 Jul;287(1):C125-34. doi: 10.1152/ajpcell.00488.2003. Epub 2004 Feb 25. | ||||
| 14 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 17 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 18 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 19 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 20 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 21 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 22 | Leclercq I, Desager JP, Horsmans Y "Inhibition of chlorzoxazone metabolism, a clinical probe for CYP2E1, by a single ingestion of watercress." Clin Pharmacol Ther 64 (1998): 144-9. [PMID: 9728894] | ||||
| 23 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 24 | Elsharkawy AM, Schwab U, McCarron B, et al. "Efavirenz induced acute liver failure requiring liver transplantation in a slow drug metaboliser." J Clin Virol 58 (2013): 331-3. [PMID: 23763943] | ||||
| 25 | Product Information. Ziagen (abacavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 26 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 27 | Canadian Pharmacists Association. | ||||
| 28 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 29 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 30 | Product Information. Clolar (clofarabine). sanofi-aventis, Bridgewater, NJ. | ||||
| 31 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 32 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 33 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 34 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 35 | Kharasch ED, Thummel KE, Mhyre J, Lillibridge JH "Single-dose disulfiram inhibition of chlorzoxazone metabolism: a clinical probe for P450 2E1." Clin Pharmacol Ther 53 (1993): 643-50. [PMID: 8513656] | ||||
| 36 | Product Information. ReVia (naltrexone). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 37 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
