Details of the Drug
General Information of Drug (ID: DMGS80V)
| Drug Name |
Levorphanol
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
levorphanol; Levorphan; Methorphinan; Aromarone; Racemethorphanum; Dromoran; Cetarin; Antalgin; Levo-Dromoran; Racemic dromoran; Methorfinan [Czech]; Levorfanolo [DCIT]; Levorphanolum; Levorfanol; Orphan; Levorfanol [INN-Spanish]; Levorphanolum [INN-Latin]; Racemorfano [INN-Spanish]; 17-Methylmorphinan-3-ol; N-Methyl-3-hydroxymorphinan; Racemorphan; Racemorphan [INN:BAN]; Racemorphane [INN-French]; Racemorphanum [INN-Latin]; (-)-N-Methylmorphinan-3-ol; Morphinan-3-ol, 17-methyl-; Levorphanal; dl-3-Hydroxy-N-methylmorphinan; DEA No; Antalgin; DEXTRORPHAN; Levodroman; Levorfanolo; Methorfinan; Racemorfano; Racemorphane; Racemorphanum; NU 2206; L-Dromoran; Levorphanol (INN); Ro 1-5431; Levo-Dromoran (TN); Dl-3-Hydroxy-N-methylmorphinan; (+)-3-Hydroxy-17-methylmorphinan; (+-)-17-Methylmorphinan-3-ol; (+-)-3-Hydroxy-N-methylmorphinan; (-)-3-Hydroxy-N-methylmorphinan; 1,3,4,9,10,10a-Hexahydro-11-methyl-2H-10,4a-iminoethanophenanthren-6-ol, dl-mixture; 1,3,4,9,10,10a-Hexahydro-6-hydroxy-2H-10,4a-(iminoethano)-11-methylphenanthrene; 3-Hydroxy-N-methylmorphinan; Dextrorphan
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
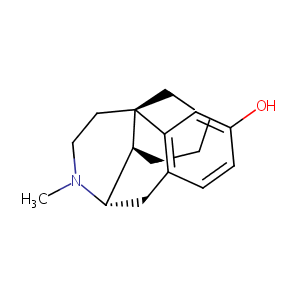 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 257.37 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Levorphanol
Coadministration of a Drug Treating the Disease Different from Levorphanol (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009336) | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Neuroprotective agents for the treatment of acute ischemic stroke. Curr Neurol Neurosci Rep. 2003 Jan;3(1):9-20. | ||||
| 6 | Comparison of the effects of dextromethorphan, dextrorphan, and levorphanol on the hypothalamo-pituitary-adrenal axis. J Pharmacol Exp Ther. 2004 May;309(2):515-22. | ||||
| 7 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 8 | Product Information. Buprenex (buprenorphine). Reckitt and Colman Pharmaceutical, Richmond, VA. | ||||
| 9 | Product Information. Apadaz (acetaminophen-benzhydrocodone). KemPharm, Inc, Coralville, IA. | ||||
| 10 | Vizcaychipi MP, Walker S, Palazzo M "Serotonin syndrome triggered by tramadol." Br J Anaesth 99 (2007): 919. [PMID: 18006535] | ||||
| 11 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 12 | Sturner WQ, Garriott JC "Deaths involving propoxyphene: a study of 41 cases over a two-year period." JAMA 223 (1973): 1125-30. [PMID: 4739371] | ||||
| 13 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 14 | Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G "Gabapentin enhances the analgesic effect of morphine in healthy volunteers." Anesth Analg 91 (2000): 185-91. [PMID: 10866910] | ||||
| 15 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 16 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 17 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 18 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 19 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 20 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 21 | Product Information. Wellbutrin XL (buPROPion). GlaxoSmithKline, Philadelphia, PA. | ||||
| 22 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 23 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
