Details of the Drug
General Information of Drug (ID: DMD1B8Z)
| Drug Name |
Dobutamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dobutamina; Dobutaminum; Dobutamine Hcl; Dobutamine [USAN]; Dobutamine hydrobromide; LY 81929; Lilly 81929; DL-dobutamine; Dobutamina [INN-Spanish]; Dobutamine Hcl in Dextrose 5%; Dobutamine(usp); Dobutaminum [INN-Latin]; Rac-dobutamine; Racemic-Dobutamine; Dobutamine (USP/INN); Dobutamine [USAN:BAN:INN]; Dobutamine Tartrate (1:1), (S-(R*,R*))-Isomer; (+-)-4-(2-((3-(p-Hydroxyphenyl)-1-methylpropyl)amino)ethyl)pyrocatechol; 3,4-dihydroxy-N-[3-(4-hydroxyphenyl)-1-methylpropyl]-beta-phenylethylamine; 4-(2-{[3-(4-hydroxyphenyl)-1-methylpropyl]amino}ethyl)benzene-1,2-diol; 4-(2-{[4-(4-hydroxyphenyl)butan-2-yl]amino}ethyl)benzene-1,2-diol; 4-[2-[4-(4-hydroxyphenyl)butan-2-ylamino]ethyl]benzene-1,2-diol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Cardiotonic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
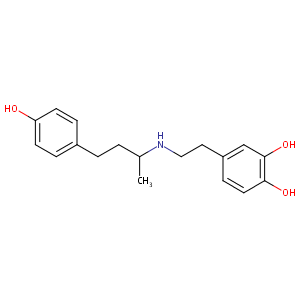 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 301.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Dobutamine
Coadministration of a Drug Treating the Disease Different from Dobutamine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
