Details of the Drug
General Information of Drug (ID: DMHTEAO)
| Drug Name |
Carvedilol
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Artist; Carvedilolum; Coreg; Coropres; Coropress; Dibloc; Dilatrend; Eucardic; Kredex; Querto; Atlana Pharma brand of carvedilol; Carvedilolum [Latin]; Coreg CR; GlaxoSmithKline brand of carvedilol; Lakeside brand of carvedilol; Roche brand of carvedilol; BM 14190; DQ 2466; SKF 105517; Artist (TN); BM-14190; Carloc (TN); Coreg (TN); DQ-2466; Dilatrend (TN); EG-P042; Eucardic (TN); BM-14-190; BM-14.190; Carvedilol, 14C-labeled; SK&F-105517; Carvedilol (JAN/USAN/INN); Carvedilol [USAN:INN:BAN:JAN]; Carvedilol, (R)-isomer; Carvedilol, (S)-isomer; Carvedilol, (+-)-isomer; (+-)-1-(Carbazol-4-yloxy)-3-((2-(o-methoxyphenoxy)ethyl)amino)-2-propanol; (+-)-1-Carbazol-4-yloxy)-3-((2-(o-methoxyphenoxy)ethyl)amino)-2-propanol; (+/-)-1-(Carbazol-4-yloxy)-3-[[2-(o-methoxyphenoxy)ethyl]amino]-2-propanol; 1-(9H-Carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)ethyl]amino]-2-propanol; 1-(9H-carbazol-4-yloxy)-3-[(2-{[2-(methyloxy)phenyl]oxy}ethyl)amino]propan-2-ol; 1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)ethylamino]propan-2-ol; 1-(9H-carbazol-4-yloxy)-3-{[2-(2-methoxyphenoxy)ethyl]amino}propan-2-ol
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Vasodilator Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
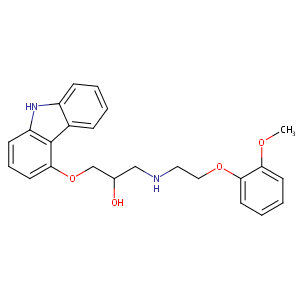 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 406.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Carvedilol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Carvedilol FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 551). | ||||
| 3 | Can beta-adrenergic blockers be used in the treatment of COVID-19 Med Hypotheses. 2020 May 5;142:109809. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Beta-blockers in the treatment of hypertension: are there clinically relevant differences Postgrad Med. 2009 May;121(3):90-8. | ||||
| 9 | Carvedilol increases the production of interleukin-12 and interferon-gamma and improves the survival of mice infected with the encephalomyocarditis virus. J Am Coll Cardiol. 2003 Jan 15;41(2):340-5. | ||||
| 10 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | ||||
| 11 | Role of cytochrome P450 2D6 genetic polymorphism in carvedilol hydroxylation in vitro. Drug Des Devel Ther. 2016 Jun 8;10:1909-16. | ||||
| 12 | In vitro identification of the human cytochrome P450 enzymes involved in the metabolism of R(+)- and S(-)-carvedilol. Drug Metab Dispos. 1997 Aug;25(8):970-7. | ||||
| 13 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 14 | The role of CYP2C9 genetic polymorphism in carvedilol O-desmethylation in vitro. Eur J Drug Metab Pharmacokinet. 2016 Feb;41(1):79-86. | ||||
| 15 | Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004 Nov;32(11):1201-8. | ||||
| 16 | Pharmacokinetic interactions study between carvedilol and some antidepressants in rat liver microsomes - a comparative study. Med Pharm Rep. 2019 Apr;92(2):158-164. | ||||
| 17 | Effect of carvedilol on plasma adiponectin concentration in patients with chronic heart failure. Circ J. 2009 Jun;73(6):1067-73. doi: 10.1253/circj.cj-08-1026. Epub 2009 Apr 14. | ||||
| 18 | Renal and cardiac function during alpha1-beta-blockade in congestive heart failure. Scand J Clin Lab Invest. 2002;62(2):97-104. doi: 10.1080/003655102753611717. | ||||
| 19 | Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006 Jun;20(3):273-82. doi: 10.1111/j.1472-8206.2006.00408.x. | ||||
| 20 | Agonist actions of "beta-blockers" provide evidence for two agonist activation sites or conformations of the human beta1-adrenoceptor. Mol Pharmacol. 2003 Jun;63(6):1312-21. doi: 10.1124/mol.63.6.1312. | ||||
| 21 | A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007 Oct 16;104(42):16657-62. doi: 10.1073/pnas.0707936104. Epub 2007 Oct 9. | ||||
| 22 | Effects of carvedilol on oxidative stress in polymorphonuclear and mononuclear cells in patients with essential hypertension. Am J Med. 2004 Apr 1;116(7):460-5. doi: 10.1016/j.amjmed.2003.10.029. | ||||
| 23 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 24 | Contribution of polymorphisms in UDP-glucuronosyltransferase and CYP2D6 to the individual variation in disposition of carvedilol. J Pharm Pharm Sci. 2006;9(1):101-12. | ||||
| 25 | Carvedilol inhibits endothelin-1 biosynthesis in cultured human coronary artery endothelial cells. J Mol Cell Cardiol. 1998 Jan;30(1):167-73. doi: 10.1006/jmcc.1997.0582. | ||||
| 26 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 27 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 28 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 29 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 30 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 31 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 32 | Chapple DJ, Clark JS, Hughes R "Interaction between atracurium and drugs used in anaesthesia." Br J Anaesth 55 Suppl 1 (1983): s17-22. [PMID: 6688011] | ||||
| 33 | Canadian Pharmacists Association. | ||||
| 34 | Product Information. Isturisa (osilodrostat). Recordati Rare Diseases Inc, Lebanon, NJ. | ||||
| 35 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 36 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 37 | Branch RA, Herman RJ "Enzyme induction and beta-adrenergic receptor blocking drugs." Br J Clin Pharmacol 17 (1984): s77-84. [PMID: 6146342] | ||||
| 38 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 39 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 40 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 41 | Amemiya M, Tabei K, Furuya H, Sakairi Y, Asano Y "Pharmacokinetics of carteolol in patients with impaired renal function." Eur J Clin Pharmacol 43 (1992): 417-21. [PMID: 1451723] | ||||
| 42 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 43 | Product Information. Vizimpro (dacomitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 44 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 45 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 46 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 47 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 48 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 49 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 50 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 51 | Dufresne RL, Weber SS, Becker RE "Bupropion hydrochloride." Drug Intell Clin Pharm 18 (1984): 957-64. [PMID: 6439541] | ||||
| 52 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 53 | Dean S, Kendall MJ, Potter S, Thompson MH, Jackson DA "Nadolol in combination with indapamide and xipamide in resistant hypertensives." Eur J Clin Pharmacol 28 (1985): 29-33. [PMID: 3987783] | ||||
| 54 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 55 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
| 56 | Product Information. Orgovyx (relugolix). Myovant Sciences, Inc., Brisbane, CA. | ||||
| 57 | Chrysant SG "Experience with terazosin administered in combination with other antihypertensive agents." Am J Med 80 (1986): 55-61. [PMID: 2872808] | ||||
| 58 | Markowitz JS, Wells BG, Carson WH "Interactions between antipsychotic and antihypertensive drugs." Ann Pharmacother 29 (1995): 603-9. [PMID: 7663034] | ||||
| 59 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 60 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 61 | Ahmad S "Metoprolol-induced delirium perpetuated by propafenone." Am Fam Physician 44 (1991): 1142,4. [PMID: 1927831] | ||||
