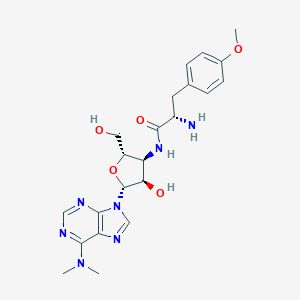
| Synonyms |
puromycin; Stylomycin; Puromycinum; Puromycine; Puromicina; UNII-4A6ZS6Q2CL; P-638; Stillomycin; CL 13900; 3123L; 4A6ZS6Q2CL; 3'-(L-alpha-Amino-p-methoxyhydrocinnamamido)-3'-deoxy-N,N-dimethyladenosine; (S)-3'-((2-Amino-3-(4-methoxyphenyl)-1-oxopropyl)amino)-3'-deoxy-N,N-dimethyladenosine; GNF-PF-2016; Puromycinum [INN-Latin]; Puromycine [INN-French]; Puromicina [INN-Spanish]; 9-{3-deoxy-3-[(O-methyl-L-tyrosyl)amino]-beta-D-xylofuranosyl}-N,N-dimethyl-9H-purin-6-amine; Bacterenomycin
|
| Chemical Identifiers |
- Formula
- C22H29N7O5
- IUPAC Name
(2S)-2-amino-N-[(2S,3S,4R,5R)-5-[6-(dimethylamino)purin-9-yl]-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]-3-(4-methoxyphenyl)propanamide - Canonical SMILES
-
CN(C)C1=NC=NC2=C1N=CN2C3C(C(C(O3)CO)NC(=O)C(CC4=CC=C(C=C4)OC)N)O
- InChI
-
InChI=1S/C22H29N7O5/c1-28(2)19-17-20(25-10-24-19)29(11-26-17)22-18(31)16(15(9-30)34-22)27-21(32)14(23)8-12-4-6-13(33-3)7-5-12/h4-7,10-11,14-16,18,22,30-31H,8-9,23H2,1-3H3,(H,27,32)/t14-,15+,16+,18+,22+/m0/s1
- InChIKey
-
RXWNCPJZOCPEPQ-NVWDDTSBSA-N
|