Details of the Drug
General Information of Drug (ID: DMGHQ37)
| Drug Name |
ICARITIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Icaritin; 118525-40-9; UNII-UFE666UELY; UFE666UELY; CHEMBL498485; 3,5,7-trihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)chromen-4-one; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-4H-1-benzopyran-4-one; Icartin; Anhydroicaritin; AC1NSXIV; Icaritin(Anhydroicaritin); MLS006010055; BIDD:ER0021; SCHEMBL4223542; Icaritin, > DTXSID00152154; MolPort-020-006-012; TUUXBSASAQJECY-UHFFFAOYSA-N; HY-N0678; ZINC14762797; BDBM50272527; AKOS015896858; CS-3679; DB12672; NCGC00345813-01; AK168251; SMR004701218
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
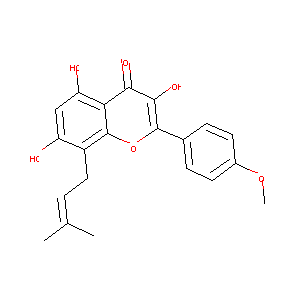 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 368.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
