Details of the Drug
General Information of Drug (ID: DMOGFIX)
| Drug Name |
Eltrombopag
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Eltrombopag [INN]; SB 497115; SB497115; SB-497115; [1,1'-Biphenyl]-3-carboxylic acid, 3'-[(2Z)-[1-(3,4-dime; Thylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-(9CI); (E)-3\'-(2-(1-(3,4-Dimethylphenyl)-3-methyl-5-oxo-1H-pyrazol-4(5H)-ylidene)hydrazinyl)-2\'-hydroxybiphenyl-3-carboxylic acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
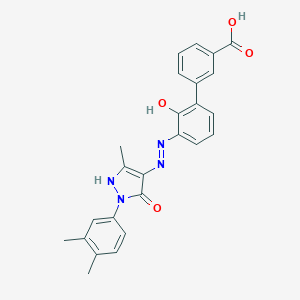 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 442.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 03 Disease of the blood or blood-forming organs | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 3B64 Thrombocytopenia | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Studied Tissue | Whole blood | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Studied Disease | Thrombocytopenia [ICD-11:3B64] | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Eltrombopag
Coadministration of a Drug Treating the Disease Different from Eltrombopag (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6961). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Early onset of abdominal venous thrombosis in a newborn with homozygous type II heparin-binding site antithrombin deficiency. Blood Coagul Fibrinolysis. 2017 Apr;28(3):264-266. doi: 10.1097/MBC.0000000000000570. | ||||
| 5 | Emerging drugs for idiopathic thrombocytopenic purpura in adults. Expert Opin Emerg Drugs. 2008 Jun;13(2):237-54. | ||||
| 6 | Pharmacokinetics and hepatic uptake of eltrombopag, a novel platelet-increasing agent. Drug Metab Dispos. 2011 Jun;39(6):1088-96. | ||||
| 7 | Assessment of the pharmacokinetic interaction between eltrombopag and lopinavir-ritonavir in healthy adult subjects. Antimicrob Agents Chemother. 2012 Jun;56(6):2846-51. | ||||
| 8 | Eltrombopag-induced acute liver failure in a pediatric patient: a pharmacokinetic and pharmacogenetic analysis. Ther Drug Monit. 2018 Aug;40(4):386-388. | ||||
| 9 | Eltrombopag for use in children with immune thrombocytopenia. Blood Adv. 2018 Feb 27;2(4):454-461. | ||||
| 10 | Allred AJ, Bowen CJ, Park JW, et al. "Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers." Br J Clin Pharmacol 72 (2011): 321-9. [PMID: 21434975] | ||||
| 11 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 12 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 13 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 14 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 15 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 16 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 17 | Product Information. Vosevi (sofosbuvir/velpatasvir/voxilaprevir). Gilead Sciences, Foster City, CA. | ||||
| 18 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 19 | Canadian Pharmacists Association. | ||||
| 20 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 21 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 22 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 23 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 24 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 25 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 26 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 27 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 28 | Product Information. Ferriprox (deferiprone). ApoPharma USA Inc, Rockville, MD. | ||||
