Details of the Drug
General Information of Drug (ID: DMTP2NJ)
| Drug Name |
Desonide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Apolar; DesOwen; Desilux; Desonate; Desonida; Desonidum; Flusemidon; Hamiltoderm; Locapred; Prednacinolone; Prenacid; Reticus; Sterax; Steroderm; Topifug; Tridesilon; Tridesonit; Verdeso; Zotinar; Desfluorotriamcinolone acetonide; D 2083; D-2083; Desonida [INN-Spanish]; Desonidum [INN-Latin]; Desowen (TN); Verdeso (TN);Verdeso Foam (TN); Desonide (USAN/INN); Desonide [USAN:INN:BAN]; Locapred, Topifug, Tridesilon, Desonide; (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one; 11beta,16alpha,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone; 11beta,21-dihydroxy-16alpha,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione; 11beta,21-dihydroxy-16alpha,17-isopropylidenedioxypregna-1,4-diene-3,20-dione; 11beta,21-dihydroxy-16alpha,17alpha-isopropylidenedioxypregna-1,4-diene-3,20-dione; 16-alpha-Hydroxyprednisole-16,17-acetonide; 16alpha,17alpha-isopropylidenedioxyprednisolone; 16alpha-hydroxyprednisole-16,17-acetonide; 16alpha-hydroxyprednisolone-16alpha,17-acetonide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
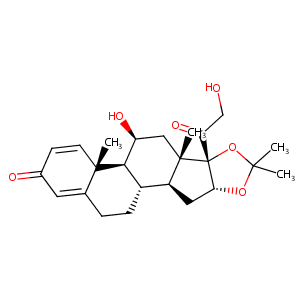 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 416.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Desonide FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7066). | ||||
| 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 4 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
