| 1 |
ClinicalTrials.gov (NCT03379051) Phase I/II Study of Venetoclax or Lenalidomide in Combination With Ublituximab and Umbralisib in Subjects With Relapsed or Refractory CLL/SLL and NHL
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2021
|
| 4 |
ClinicalTrials.gov (NCT02793583) Study to Assess the Efficacy and Safety of Ublituximab + Umbralisib With or Without Bendamustine and Umbralisib Alone in Patients With Previously Treated Non-Hodgkins Lymphoma (UNITY-NHL). U.S. National Institutes of Health.
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Venclexta FDA label
|
| 7 |
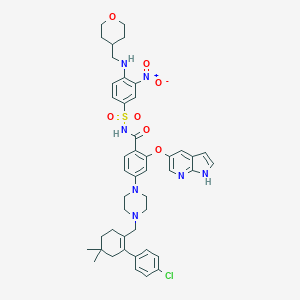
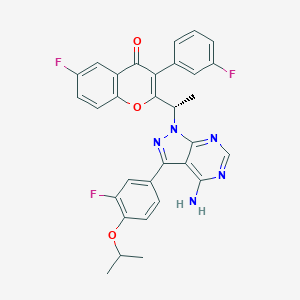
ZCL-082, a boron-containing compound, induces apoptosis of non-Hodgkin's lymphoma via targeting p90 ribosomal S6 kinase 1/NF-B signaling pathway. Chem Biol Interact. 2022 Jan 5;351:109770. doi: 10.1016/j.cbi.2021.109770. Epub 2021 Nov 30.
|
| 8 |
Genomics and drug profiling of fatal TCF3-HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat Genet. 2015 Sep;47(9):1020-1029. doi: 10.1038/ng.3362. Epub 2015 Jul 27.
|
| 9 |
HSP90 Inhibitor PU-H71 in Combination with BH3-Mimetics in the Treatment of Acute Myeloid Leukemia. Curr Issues Mol Biol. 2023 Aug 23;45(9):7011-7026. doi: 10.3390/cimb45090443.
|
| 10 |
Superior efficacy of cotreatment with BET protein inhibitor and BCL2 or MCL1 inhibitor against AML blast progenitor cells. Blood Cancer J. 2019 Jan 15;9(2):4. doi: 10.1038/s41408-018-0165-5.
|
| 11 |
Prospective Drug Candidates as Human Multidrug Transporter ABCG2 Inhibitors: an In Silico Drug Discovery Study. Cell Biochem Biophys. 2021 Jun;79(2):189-200. doi: 10.1007/s12013-021-00985-y. Epub 2021 May 5.
|
| 12 |
Statin-induced Mitochondrial Priming Sensitizes Multiple Myeloma Cells to BCL2 and MCL-1 Inhibitors. Cancer Res Commun. 2023 Dec 8;3(12):2497-2509. doi: 10.1158/2767-9764.CRC-23-0350.
|
|
|
|
|
|
|