Details of the Drug
General Information of Drug (ID: DMBL79I)
| Drug Name |
Proguanil
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bigumal; Chlorguanid; Chlorguanide; Chloriguane; Chloroguanide; Paludrin; Proguanile; Proguanilum; Proguanile [DCIT]; Diguanyl (hydrochloride); Drinupal (hydrochloride); Paludrine (TN); Paludrine (hydrochloride); Palusil (hydrochloride); Proguanil [INN:BAN]; Proguanilum [INN-Latin]; RP-3359; Tirian (hydrochloride); M-4888 (hydrochloride); SN-12837 (hydrochloride); N1-p-Chlorophenyl-N5-isopropylbiguanide; N-(4-Chlorophenyl)-N'-(1-methylethyl)imidodicarbonimidic diamide; N-(4-Chlorophenyl)-N'-(isopropyl)-imidodicarbonimidic diamide; N-(4-chlorophenyl)-N'-(propan-2-yl)imidodicarbonimidic diamide; (1E)-1-[amino-(4-chloroanilino)methylidene]-2-propan-2-ylguanidine; 1-(p-Chlorophenyl)-5-isopropylbiguanide; 1-Isopropyl-5-(4-chlorophenyl)biguanide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antimalarials
|
||||||||||||||||||||||
| Affected Organisms |
Plasmodium
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
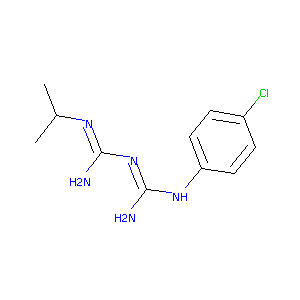 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 253.73 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Malaria | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1F40-1F45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Proguanil (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
