Details of the Drug
General Information of Drug (ID: DMMU6BJ)
| Drug Name |
Sodium bicarbonate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acidosan; Colovage; Glycoprep; Golytely; Jusonin; Meylon; Natriumhydrogenkarbonat; Natron; Neut; Nulytely; Soludal; BSS plus; Baking soda; Bicarbonate of soda; Carbonic acid monosodium salt; Gripe water; Lithium bicarbonate; Monosodium hydrogen carbonate; Natrii hydrogencarbonas; Natrium bicarbonicum; Natrium hydrogencarbonicum; Natrum bicarbonicum; Sandoz sodium bicarbonate; Sel De vichy; Soda Mint; Sodium acid carbonate; Sodium bicarbonate in plastic container; Sodium bicarbonate liquid concentrate; Sodium bicarbonate solution; Sodium hydrocarbonate; Sodium hydrogen carbonate; Sodium hydrogencarbonate; NaHCO3; Bicarbonate, Sodium; Carbonic acid, monosodium salt; Co-lav; Col-evac; Go-evac; Hema BP-38; Hydrogen Carbonate, Sodium; Meylon (TN); Neut (TN); Peg-lyte; Soda (van); Soda, Baking; Sodium bicarbonate [USAN:JAN]; Carbonic acid sodium salt (1:1); E-Z-EM Prep Lyte; Sodium bicarbonate (1:1); Sodium bicarbonate (JP15/USP); Sodium carbonate (Na(HCO3)); 800 Sodium Bicarbonate Powder
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antidiarrheals
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
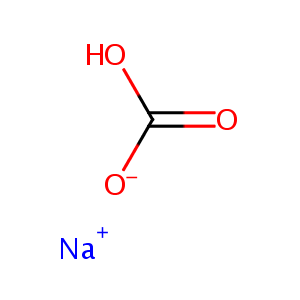 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 84.007 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Metabolic acidosis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5C73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Sodium bicarbonate (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4507). | ||||
|---|---|---|---|---|---|
| 2 | Acute renal failure. Clin Evid (Online). 2008 Sep 3;2008. pii: 2001. | ||||
| 3 | Management of cocaine-induced cardiac arrhythmias due to cardiac ion channel dysfunction. Clin Toxicol (Phila). 2009 Jan;47(1):14-23. | ||||
| 4 | The sodium bicarbonate cotransporter: structure, function, and regulation. Semin Nephrol. 2006 Sep;26(5):352-60. | ||||
| 5 | Molecular mechanisms of electrogenic sodium bicarbonate cotransport: structural and equilibrium thermodynamic considerations. J Membr Biol. 2004 Jan 15;197(2):77-90. | ||||
| 6 | A novel sodium bicarbonate cotransporter-like gene in an ancient duplicated region: SLC4A9 at 5q31. Genome Biol. 2001;2(4):RESEARCH0011. | ||||
| 7 | The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem. 2010 Aug 6;285(32):24432-8. | ||||
| 8 | G418-mediated ribosomal read-through of a nonsense mutation causing autosomal recessive proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2008 Sep;295(3):F633-41. doi: 10.1152/ajprenal.00015.2008. Epub 2008 Jul 9. | ||||
| 9 | Honig PK, Gillespie BK "Clinical significance of pharmacokinetic drug interactions with over-the-counter (OTC) drugs." Clin Pharmacokinet 35 (1998): 167-71. [PMID: 9784931] | ||||
| 10 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 11 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 12 | Product Information. Nerlynx (neratinib). Puma Biotechnology, Inc., Los Angeles, CA. | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Allen MD, Greenblatt DJ, Harmatz JS, Smith TW "Effect of magnesium--aluminum hydroxide and kaolin--pectin on absorption of digoxin from tablets and capsules." J Clin Pharmacol 21 (1981): 26-30. [PMID: 7012189] | ||||
| 15 | Product Information. Harvoni (ledipasvir-sofosbuvir). Gilead Sciences, Foster City, CA. | ||||
| 16 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 17 | Product Information. Edurant (rilpivirine). Tibotec Pharmaceuticals, Titusville, NJ. | ||||
| 18 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 19 | Chun AH, Carrigan PJ, Hoffman DJ, Kershner RP, Stuart JD "Effect of antacids on absorption of clorazepate." Clin Pharmacol Ther 22 (1977): 329-35. [PMID: 19188] | ||||
| 20 | Product Information. Zykadia (ceritinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 21 | Product Information. Retevmo (selpercatinib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 22 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 23 | Anggard E, Jonsson LE, Hogmark AL, Gunne LM "Amphetamine metabolism in amphetamine psychosis." Clin Pharmacol Ther 14 (1973): 870-80. [PMID: 4729903] | ||||
| 24 | Balali-Mood M, Prescott LF "Failure of alkaline diuresis to enhance diflunisal elimination." Br J Clin Pharmacol 10 (1980): 163-5. [PMID: 7426277] | ||||
| 25 | Product Information. Adempas (riociguat). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 26 | Product Information. Inlyta (axitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
