Details of the Drug
General Information of Drug (ID: DMBSXI0)
| Drug Name |
Dienestrol
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Agaldog; Cycladiene; Dehydrostilbestrol; Dehydrostilboestrol; Dienesterol; Dienestrolo; Dienestrolum; Dienoestrol; Dienol; Dinestrol; Dinovex; Estragard; Estraguard; Estrodienol; Estroral; Follidiene; Follormon; Gynefollin; Hormofemin; Isodienestrol; Oestrasid; Oestrodiene; Oestrodienol; Oestroral; Oestrovis; Restrol; Retalon; Sexadien; Synestrol; Teserene; Willnestrol; Dienestrolo [DCIT]; Dienoestrol [Nonsteroidal oestrogens]; Dienoestrol bp; Alpha-Dienestrol; DV (TN); Dienestrolum [INN-Latin]; Para-Dien; Restrol, Dienestrol; Dienestrol (E,E); Dienestrol (USP/INN); Dienestrol, (E,E)-Isomer; P,p'-(Diethylideneethylene)diphenol; Para,para'-(Diethylideneethylene)diphenol; Di(p-oxyphenyl)-2,4-hexadiene; Di(para-oxyphenyl)-2,4-hexadiene; Phenol, 4,4'-(1,2-diethylidene-1,2-ethanediyl)bis-, (E,E-(9CI); (E,E)-Dienestrol; 3,4-Bis(4-hydroxyphenyl)-2,4-hexadiene; 3,4-Bis(p-hydroxyphenyl)-2,4-hexadiene; 3,4-Bis(para-hydroxyphenyl)-2,4-hexadiene; 4,4'-(1,2-Diethylidene-1,2-ethanediyl)bisphenol; 4,4'-(2E,4E)-hexa-2,4-diene-3,4-diyldiphenol; 4,4'-(Diethylideneethylene)diphenol; 4,4'-Dihydroxy-gamma,delta-diphenyl-beta,delta-hexadiene; 4,4'-Hydroxy-gamma,delta-diphenyl-beta,delta-hexadiene; 4,4'-hexa-2,4-diene-3,4-diyldiphenol; 4-[(2E,4E)-4-(4-hydroxyphenyl)hexa-2,4-dien-3-yl]phenol
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Estrogens
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
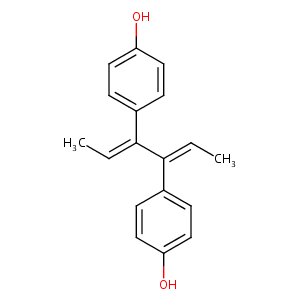 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 266.3 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.4 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Dienestrol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
