Details of the Drug
General Information of Drug (ID: DMPMYE8)
| Drug Name |
Miconazole
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dactarin; MCZ; Miconazol; Miconazolo; Miconazolum; Micozole; Minostate; Monistat; Vusion; Zimycan; Daktarin IV; Miconasil Nitrate; Miconazole nitrate salt; Miconazolo [DCIT]; Monistat IV; MJR 1762; Miconazole 3; Miconazole 3 Combination Pack; Miconazole 7 Combination Pack; Monazole 7; Monistat 1 Combination Pack; Monistat 3 Vaginal Ovules; Monistat 5 Tampon; Monistat 7 Vaginal Suppositories; R 18134; Aflorix(nitrate); Albistat(nitrate); Andergin(nitrate); Conofite(nitrate); Daktarin (TN); Femizol (TN); Femizol-M; Florid(nitrate); Lotrimin AF(nitrate); Micantin (nitrate); Micatin (TN); Miconazex (TN); Miconazol [INN-Spanish]; Miconazole-7; Miconazolum [INN-Latin]; Monista (nitrate); Monistat (TN); Monistat 3 Dual-Pak; Monistat 7 Dual-Pak; Monistat Dual-PAK; Monistat iv (TN); Monistat-Derm; Novo-Miconazole Vaginal Ovules; Epi-Monistat(nitrate); Gyno-Daktar(nitrate); Monistat-Derm (TN); R-14,889; Miconazole (JP15/USP/INN); Miconazole [USAN:BAN:INN:JAN]; Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl) methoxy)ethyl)-(9CI); Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-(9CI); (+-)-1-(2,4-Dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)imidazole; 1-(2,4-Dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)imidazole; 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl) imidazole; 1-[2,4-Dichloro-beta-([2,4-dichloro-benzyl]oxy)phenethyl]imidazole; 1-[2-(2,4-Dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-1H-imidazole; 1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole; 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]imidazole; 1-[2-(2,4-dichlorophenyl)-2-{[(2,4-dichlorophenyl)methyl]oxy}ethyl]-1H-imidazole; 1-{2-[(2,4-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Fungi, yeast and protozoans
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
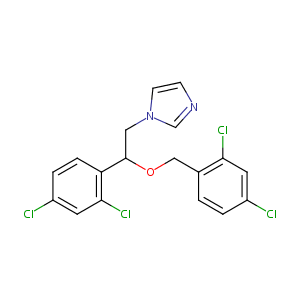 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 416.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Miconazole (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Miconazole FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2449). | ||||
| 3 | FDA Approved Drug Products: Oravig (miconazole) buccal tablet | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Stevens RE, Konsil J, Verrill SS, Roy P, Desai PB, Upmalis DH, Cone FL: Bioavailability study of a 1200 mg miconazole nitrate vaginal ovule in healthy female adults. J Clin Pharmacol. 2002 Jan;42(1):52-60. doi: 10.1177/0091270002042001006. | ||||
| 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 9 | Inhibition of cytochrome P450 enzymes participating in p-nitrophenol hydroxylation by drugs known as CYP2E1 inhibitors. Chem Biol Interact. 2004 Apr 15;147(3):331-40. | ||||
| 10 | Direct stimulation of adenylyl cyclase 9 by the fungicide imidazole miconazole. Naunyn Schmiedebergs Arch Pharmacol. 2019 Apr;392(4):497-504. doi: 10.1007/s00210-018-01610-1. Epub 2019 Jan 3. | ||||
| 11 | Comparative assessment of the inhibition of recombinant human CYP19 (aromatase) by azoles used in agriculture and as drugs for humans. Endocr Res. 2004 Aug;30(3):387-94. | ||||
| 12 | Miconazole induces protective autophagy in bladder cancer cells. Environ Toxicol. 2021 Feb;36(2):185-193. doi: 10.1002/tox.23024. Epub 2020 Sep 27. | ||||
| 13 | In vitro inhibitory effects of asiaticoside and madecassoside on human cytochrome P450. Toxicol In Vitro. 2011 Jun;25(4):890-6. | ||||
| 14 | The novel azole R126638 is a selective inhibitor of ergosterol synthesis in Candida albicans, Trichophyton spp., and Microsporum canis. Antimicrob Agents Chemother. 2004 Sep;48(9):3272-8. | ||||
| 15 | Azole antimycotics differentially affect rifampicin-induced pregnane X receptor-mediated CYP3A4 gene expression. Drug Metab Dispos. 2008 Feb;36(2):339-48. | ||||
| 16 | Anti-mycotics suppress interleukin-4 and interleukin-5 production in anti-CD3 plus anti-CD28-stimulated T cells from patients with atopic dermatitis. J Invest Dermatol. 2001 Dec;117(6):1635-46. doi: 10.1046/j.0022-202x.2001.01566.x. | ||||
| 17 | Differential azole antifungal efficacies contrasted using a Saccharomyces cerevisiae strain humanized for sterol 14 alpha-demethylase at the homolo... Antimicrob Agents Chemother. 2008 Oct;52(10):3597-603. | ||||
| 18 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 19 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 20 | Product Information. ORAVIG (miconazole). Strativa Pharmaceuticals, a Division of Par Pharmaceuticals, Inc., Woodcliff Lake, NJ. | ||||
| 21 | Heinig R, Adelmann HG, Ahr G "The effect of ketoconazole on the pharmacokinetics, pharmacodynamics and safety of nisoldipine." Eur J Clin Pharmacol 55 (1999): 57-60. [PMID: 10206086] | ||||
| 22 | Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN "Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole." Ann Pharmacother 38 (2004): 46-9. [PMID: 14742792] | ||||
| 23 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 24 | Product Information. Vraylar (cariprazine). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 25 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 26 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 27 | Devenport MH, Crook D, Wynn V, Lees LJ "Metabolic effects of low-dose fluconazole in healthy female users and non-users of oral contraceptives." Br J Clin Pharmacol 27 (1989): 851-9. [PMID: 2547410] | ||||
| 28 | Product Information. Jevtana (cabazitaxel). sanofi-aventis , Bridgewater, NJ. | ||||
| 29 | Product Information. Opsumit (macitentan). Actelion Pharmaceuticals US Inc, South San Francisco, CA. | ||||
| 30 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 31 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 32 | Ariyaratnam S, Thakker NS, Sloan P, Thornhill MH "Potentiation of warfarin anticoagulant activity by miconazole oral gel." BMJ 314 (1997): 349. [PMID: 9040331] | ||||
| 33 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 34 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 35 | Product Information. Viibryd (vilazodone). Trovis Pharmaceuticals LLC, New Haven, CT. | ||||
| 36 | Product Information. Serzone (nefazodone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 37 | Product Information. Celexa (citalopram). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 38 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 39 | Lazar JD, Wilner KD "Drug interactions with fluconazole." Rev Infect Dis 12 Suppl 3 (1990): s327-33. [PMID: 2330488] | ||||
| 40 | Product Information. Osphena (ospemifene). Shionogi USA Inc, Florham Park, NJ. | ||||
| 41 | Rolan PE, Somogyi AA, Drew MJ, et al "Phenytoin intoxication during treatment with parenteral miconazole." Br Med J (Clin Res Ed) 287 (1983): 1760. [PMID: 6416579] | ||||
| 42 | Product Information. VESIcare (solifenacin). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 43 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 44 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 45 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 46 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 47 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 48 | Canadian Pharmacists Association. | ||||
| 49 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 50 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 51 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 52 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 53 | Product Information. Xalkori (crizotinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 54 | Product Information. Zykadia (ceritinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 55 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 56 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 57 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 58 | Product Information. Imbruvica (ibrutinib). Pharmacyclics Inc, Sunnyvale, CA. | ||||
| 59 | Product Information. Iclusig (ponatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 60 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 61 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 62 | Hamberg P, Woo MM, Chen LC, et al. "Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor." Cancer Chemother Pharmacol 68 (2011): 805-13. [PMID: 21706316] | ||||
| 63 | Product Information. Istodax (romidepsin). Gloucester Pharmaceuticals, Cambridge, MA. | ||||
| 64 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 65 | Product Information. Lynparza (olaparib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 66 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 67 | Caballero-Granado FJ, Viciana P, Cordero E, Gomez-Vera MJ, del Nozal M, Lopez-Cortes LF "Ergotism related to concurrent administration of ergotamine tartrate and ritonavir in an AIDS patient." Antimicrob Agents Chemother 41 (1997): 1207. [PMID: 9145904] | ||||
| 68 | Product Information. Xtandi (enzalutamide). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 69 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 70 | Product Information. Rapaflo (silodosin). Watson Pharmaceuticals, Corona, CA. | ||||
| 71 | Product Information. Duagen (dutasteride). GlaxoSmithKline Healthcare, Pittsburgh, PA. | ||||
| 72 | Product Information. Letairis (ambrisentan). Gilead Sciences, Foster City, CA. | ||||
| 73 | Product Information. Torisel (temsirolimus). Wyeth-Ayerst Laboratories, Philadelphia, PA. | ||||
| 74 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 75 | Edsbacker S, Andersson T "Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn's disease." Clin Pharmacokinet 43 (2004): 803-21. [PMID: 15355126] | ||||
| 76 | DeVane CL, Nemeroff CB "Clinical pharmacokinetics of quetiapine - An atypical antipsychotic." Clin Pharmacokinet 40 (2001): 509-22. [PMID: 11510628] | ||||
| 77 | Product Information. Abilify (aripiprazole). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 78 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 79 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 80 | Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K "Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60 year old Caucasian woman with ovarian carcinoma." Br J Clin Pharmacol (2015):. [PMID: 26446447] | ||||
| 81 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 82 | Product Information. Cometriq (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 83 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
