Details of the Drug
General Information of Drug (ID: DMCR1MV)
| Drug Name |
Itraconazole
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Canadiol; Hyphanox; ITCZ; ITZ; Intraconazole; Itraconazol; Itraconazolum; Itrizole; Oriconazole; Orungal; Prokanazol; Sempera; Spherazole; Sporal; Sporanos; Sporanox; Sporonox; Triasporn; Itraconazol [Spanish]; Itraconazole oral solution; Itraconazolum [Latin]; R 51211; Cis-Itraconazole; Itraconazole & Bovine Lactoferrin; Itraconazole & Nyotran; Itrizole (TN); R-51211; Sporanox (TN); Itraconazole & Nyotran(Liposomal Nystatin); Itraconazole (JAN/USAN); Oriconazole, R51211, Sporanox; Itraconazole [USAN:BAN:INN:JAN]; (+-)-1-sec-Butyl-4-(p-(4-(p-(((2R*,4S*)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-1-piperazinyl)phenyl)-delta(sup 2)-1,2,4-triazolin-5-one; (1)-cis-4-(4-(4-(4-((2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-2,4-dihydro-2-sec-butyl-3H-1,2,4-triazol-3-one; 2-(butan-2-yl)-4-{4-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]phenyl}-2,4-dihydro-3H-1,2,4-triazol-3-one; 2-butan-2-yl-4-[4-[4-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one; 2-butan-2-yl-4-[4-[4-[4-[[(2S,4R)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one; 2-butan-2-yl-4-[4-[4-[4-[[2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one; 3H-1,2,4-Triazol-3-one, 4-[4-[4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-pipera-zinyl]phenyl]-2,4-dihydro-2-(1-methylpropyl); 4-(4-{4-[4-({[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methyl}oxy)phenyl]piperazin-1-yl}phenyl)-2-(1-methylpropyl)-2,4-dihydro-3H-1,2,4-triazol-3-one
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Fungi, yeast and protozoans
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
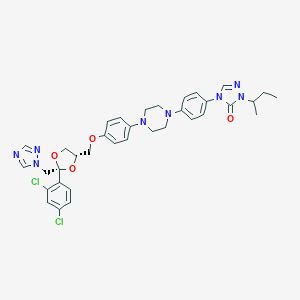 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 705.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Itraconazole (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
| DIG |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Formulation |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
| 1 | Itraconazole FDA Label | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 076104. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 7 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 8 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | ||||
| 9 | Effects of itraconazole and tandospirone on the pharmacokinetics of perospirone. Ther Drug Monit. 2006 Feb;28(1):73-5. | ||||
| 10 | Investigation into UDP-glucuronosyltransferase (UGT) enzyme kinetics of imidazole- and triazole-containing antifungal drugs in human liver microsomes and recombinant UGT enzymes. Drug Metab Dispos. 2010 Jun;38(6):923-9. | ||||
| 11 | The novel azole R126638 is a selective inhibitor of ergosterol synthesis in Candida albicans, Trichophyton spp., and Microsporum canis. Antimicrob Agents Chemother. 2004 Sep;48(9):3272-8. | ||||
| 12 | Inhibition of 11-hydroxysteroid dehydrogenase 2 by the fungicides itraconazole and posaconazole. Biochem Pharmacol. 2017 Apr 15;130:93-103. doi: 10.1016/j.bcp.2017.01.010. Epub 2017 Jan 25. | ||||
| 13 | Clinical pharmacokinetic monitoring of itraconazole is warranted in only a subset of patients. Ther Drug Monit. 2005 Jun;27(3):322-33. doi: 10.1097/01.ftd.0000150135.22645.ea. | ||||
| 14 | Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500. | ||||
| 15 | Induction of cytochrome P450 1A1 by ketoconazole and itraconazole but not fluconazole in murine and human hepatoma cell lines. Toxicol Sci. 2007 May;97(1):32-43. | ||||
| 16 | Itraconazole cis-diastereoisomers activate aryl hydrocarbon receptor AhR and pregnane X receptor PXR and induce CYP1A1 in human cell lines and human hepatocytes. Toxicology. 2017 May 15;383:40-49. | ||||
| 17 | Anti-mycotics suppress interleukin-4 and interleukin-5 production in anti-CD3 plus anti-CD28-stimulated T cells from patients with atopic dermatitis. J Invest Dermatol. 2001 Dec;117(6):1635-46. doi: 10.1046/j.0022-202x.2001.01566.x. | ||||
| 18 | Differential azole antifungal efficacies contrasted using a Saccharomyces cerevisiae strain humanized for sterol 14 alpha-demethylase at the homolo... Antimicrob Agents Chemother. 2008 Oct;52(10):3597-603. | ||||
| 19 | Azole antimycotics differentially affect rifampicin-induced pregnane X receptor-mediated CYP3A4 gene expression. Drug Metab Dispos. 2008 Feb;36(2):339-48. | ||||
| 20 | Brass C, Galgiani JN, Blaschke TF, et al "Disposition of ketoconazole, an oral antifungal, in humans." Antimicrob Agents Chemother 21 (1982): 151-8. [PMID: 6282204] | ||||
| 21 | Product Information. Cleocin (clindamycin). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 22 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 23 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 24 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 25 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 26 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 27 | Product Information. Olinvyk (oliceridine). Trevena Inc, Chesterbrook, PA. | ||||
| 28 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 29 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 30 | Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN "Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole." Ann Pharmacother 38 (2004): 46-9. [PMID: 14742792] | ||||
| 31 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 32 | Auclair B, Berning SE, Huitt GA, Peloquin CP "Potential interaction between itraconazole and clarithromycin." Pharmacotherapy 19 (1999): 1439-44. [PMID: 10600094] | ||||
| 33 | Product Information. Vraylar (cariprazine). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 34 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 35 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 36 | Product Information. Ixempra (ixabepilone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 37 | Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D "Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects." Br J Clin Pharmacol 71 (2011): 522-7. [PMID: 21395644] | ||||
| 38 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 39 | Devenport MH, Crook D, Wynn V, Lees LJ "Metabolic effects of low-dose fluconazole in healthy female users and non-users of oral contraceptives." Br J Clin Pharmacol 27 (1989): 851-9. [PMID: 2547410] | ||||
| 40 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 41 | Product Information. Opsumit (macitentan). Actelion Pharmaceuticals US Inc, South San Francisco, CA. | ||||
| 42 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 43 | Product Information. Breo Ellipta (fluticasone-vilanterol). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 44 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 45 | Product Information. Fetzima (levomilnacipran). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 46 | Product Information. Ofev (nintedanib). Boehringer Ingelheim, Ridgefield, CT. | ||||
| 47 | Product Information. Celexa (citalopram). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 48 | Product Information. Rexulti (brexpiprazole). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 49 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 50 | Lazar JD, Wilner KD "Drug interactions with fluconazole." Rev Infect Dis 12 Suppl 3 (1990): s327-33. [PMID: 2330488] | ||||
| 51 | Product Information. Osphena (ospemifene). Shionogi USA Inc, Florham Park, NJ. | ||||
| 52 | Product Information. Ingrezza (valbenazine). Neurocrine Biosciences, Inc., San Diego, CA. | ||||
| 53 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 54 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 55 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 56 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 57 | Bottiger Y, Tybring G, Gotharson E, Bertilsson L "Inhibition of the sulfoxidation of omeprazole by ketoconazole in poor and extensive metabolizers of S-mephenytoin." Clin Pharmacol Ther 62 (1997): 384-91. [PMID: 9357389] | ||||
| 58 | Akdag I, Ersoy A, Kahvecioglu S, Gullulu M, Dilek K "Acute colchicine intoxication during clarithromycin administration in patients with chronic renal failure." J Nephrol 19 (2006): 515-7. [PMID: 17048210] | ||||
| 59 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 60 | Abadie-Kemmerly S, Pankey GA, Dalvisio JR "Failure of ketoconazole treatment of blastomyces dermatidis due to interaction of isoniazid and rifampin." Ann Intern Med 109 (1988): 844-5. [PMID: 3190036] | ||||
| 61 | Product Information. VFEND (voriconazole). Pfizer U.S. Pharmaceuticals, New York, NY. | ||||
| 62 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 63 | Polk RE, Crouch MA, Israel DS, et al. "Pharmacokinetic interaction between ketoconazole and amprenavir after single doses in healthy men." Pharmacotherapy 19 (1999): 1378-84. [PMID: 10600086] | ||||
| 64 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 65 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 66 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 67 | Product Information. Edurant (rilpivirine). Tibotec Pharmaceuticals, Titusville, NJ. | ||||
| 68 | Product Information. Selzentry (maraviroc). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 69 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 70 | Canadian Pharmacists Association. | ||||
| 71 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 72 | Product Information. Xerava (eravacycline). Tetraphase Pharmaceuticals, Inc, Watertown, MA. | ||||
| 73 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 74 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 75 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 76 | Product Information. Viberzi (eluxadoline). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 77 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 78 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 79 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 80 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 81 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 82 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 83 | Abernethy DR, Wesche DL, Barbey JT, et al. "Stereoselective halofantrine disposition and effect: concentration-related QTc prolongation." Br J Clin Pharmacol 51 (2001): 231-7. [PMID: 11298069] | ||||
| 84 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 85 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 86 | Product Information. Venclexta (venetoclax). AbbVie US LLC, North Chicago, IL. | ||||
| 87 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 88 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 89 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 90 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 91 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 92 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 93 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 94 | Hamberg P, Woo MM, Chen LC, et al. "Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor." Cancer Chemother Pharmacol 68 (2011): 805-13. [PMID: 21706316] | ||||
| 95 | Product Information. Istodax (romidepsin). Gloucester Pharmaceuticals, Cambridge, MA. | ||||
| 96 | Product Information. Jakafi (ruxolitinib). Incyte Corporation, Wilmington, DE. | ||||
| 97 | Product Information. Orlaam (levomethadyl acetate) Roxanne Laboratories Inc, Columbus, OH. | ||||
| 98 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 99 | Product Information. Zejula (niraparib). Tesaro Inc., Waltham, MA. | ||||
| 100 | Product Information. Nourianz (istradefylline). Kyowa Kirin, Inc, Bedminster, NJ. | ||||
| 101 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 102 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 103 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 104 | Blanche P, Rigolet A, Gombert B, Ginsburg C, Salmon D, Sicard D "Ergotism related to a single dose of ergotamine tartrate in an AIDS patient treated with ritonavir." Postgrad Med J 75 (1999): 546-7. [PMID: 10616689] | ||||
| 105 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 106 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
| 107 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 108 | Product Information. Orgovyx (relugolix). Myovant Sciences, Inc., Brisbane, CA. | ||||
| 109 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 110 | Product Information. Letairis (ambrisentan). Gilead Sciences, Foster City, CA. | ||||
| 111 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 112 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 113 | DeVane CL, Nemeroff CB "Clinical pharmacokinetics of quetiapine - An atypical antipsychotic." Clin Pharmacokinet 40 (2001): 509-22. [PMID: 11510628] | ||||
| 114 | Product Information. Abilify (aripiprazole). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 115 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 116 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 117 | Martin P, Oliver S, Robertson J, Kennedy SJ, Read J, Duvauchelle T "Pharmacokinetic drug interactions with vandetanib during cadministration with rifampicin or itraconazole." Drugs R D 11 (2011): 37-51. [PMID: 21410294] | ||||
| 118 | Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K "Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60 year old Caucasian woman with ovarian carcinoma." Br J Clin Pharmacol (2015):. [PMID: 26446447] | ||||
| 119 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 120 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
| 121 | Arrington-Sanders R, Hutton N, Siberry GK "Ritonavir-fluticasone interaction causing Cushing syndrome in HIV-infected children and adolescents." Pediatr Infect Dis J 25 (2006): 1044-1048. [PMID: 17072128] | ||||
| 122 | Product Information. Rythmol SR (propafenone). GlaxoSmithKline, Research Triangle Park, NC. | ||||
