Details of the Drug
General Information of Drug (ID: DMG6Q45)
| Drug Name |
TDZD-8
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
327036-89-5; 4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione; GSK-3beta Inhibitor I; TDZD 8; 1,2,4-Thiadiazolidine-3,5-dione, 2-methyl-4-(phenylmethyl)-; MFCD04973552; NP 01139; AK-48153; 4-Benzyl-2-methyl-[1,2,4]thiadiazolidine-3,5-dione; 1,2,4-Thiadiazolidine-3,5-dione,2-methyl-4-(phenylmethyl)-; GSK-3 Inhibitor I; SCHEMBL139834; GTPL5977; CHEMBL284861; BDBM7781; CTK4G9152; ZINC27361; AOB6176; EX-A109; DTXSID30399590; JDSJDASOXWCHPN-UHFFFAOYSA-N; MolPort-003-844-896; HMS3229G12; A Inhibitor I, TDZD-8
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
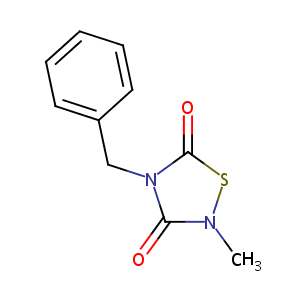 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 222.27 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
