Details of the Drug
General Information of Drug (ID: DMRJSP8)
| Drug Name |
Orlistat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
orlistat; 96829-58-2; Tetrahydrolipstatin; Xenical; Alli; Orlipastat; (-)-Tetrahydrolipstatin; Orlipastatum; Orlipastatum [INN-Latin]; THLP; Ro-18-0647; UNII-95M8R751W8; C29H53NO5; Ro 18-0647/002; N-Formyl-L-leucine (1S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester; (2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl (2S)-2-formamido-4-methylpentanoate; Orlistat (Alli, Xenical); MLS002207022; [(2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl] (2S)-2-formamido-4-methylpentanoate; CHEMBL175247; Alli; GlaxoSmithKline brand of orlistat; Roche brand of orlistat; Alli (TN); Hoffmann-La Roche brand of orlistat; KS-1183; Orlistat [USAN:INN]; R-212; Xenical (TN); Orlistat (USAN/INN); Ro 18-0647/008; Alli, Xenical, Tetrahydrolipstatin, Orlistat; N-Formyl-L-leucine, ester with (3S,4S)-3-hexyl-4-((2S)-2-hydroxytridecyl)-2-oxetanone; L-Leucine, N-formyl-, (1S)-1-(((2S,3S)-3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl ester; L-Leucine,N-formyl-, (1S)-1-(((2S,3S)-3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl ester; 1-((3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl-2-formamido-4-methylvalerate; TETRAHYDROLIPSTATIN
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiobesity Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammalsMouseRat
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
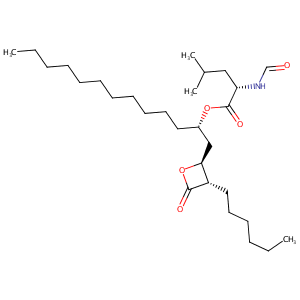 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 495.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 10 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 23 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Obesity | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5B81 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Orlistat (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | FDA Approved Drug Products: Xenical (orlistat) capsules | ||||
| 4 | Metabolic profiles of minimally absorbed orlistat in obese/overweight volunteers. J Clin Pharmacol. 1996 Nov;36(11):1006-11. doi: 10.1177/009127009603601104. | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. J Med Chem. 2008 Nov 13;51(21):6970-9. | ||||
| 7 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 8 | Diosgenin and 4-Hydroxyisoleucine from Fenugreek Are Regulators of Genes Involved in Lipid Metabolism in The Human Colorectal Cancer Cell Line SW480. Cell J. 2021 Jan;22(4):514-522. doi: 10.22074/cellj.2021.6751. Epub 2020 Apr 22. | ||||
| 9 | Profiling the Tox21 Chemical Collection for Acetylcholinesterase Inhibition. Environ Health Perspect. 2021 Apr;129(4):47008. doi: 10.1289/EHP6993. Epub 2021 Apr 12. | ||||
| 10 | Orlistat Displays Antitumor Activity and Enhances the Efficacy of Paclitaxel in Human Hepatoma Hep3B Cells. Chem Res Toxicol. 2019 Feb 18;32(2):255-264. doi: 10.1021/acs.chemrestox.8b00269. Epub 2019 Jan 22. | ||||
| 11 | Decrements in the thrombin activatable fibrinolysis inhibitor (TAFI) levels in association with orlistat treatment in obesity. Clin Appl Thromb Hemost. 2006 Jul;12(3):364-8. doi: 10.1177/1076029606291403. | ||||
| 12 | Antitumor effect of orlistat, a fatty acid synthase inhibitor, is via activation of caspase-3 on human colorectal carcinoma-bearing animal. Biomed Pharmacother. 2011 Jul;65(4):286-92. doi: 10.1016/j.biopha.2011.02.016. Epub 2011 Jun 12. | ||||
| 13 | Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7-36)-amide-1, cholecystokinin, and peptide YY concentrations. J Clin Endocrinol Metab. 2008 Oct;93(10):3995-8. doi: 10.1210/jc.2008-0924. Epub 2008 Jul 22. | ||||
| 14 | Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007 Feb 1;67(3):1262-9. doi: 10.1158/0008-5472.CAN-06-1794. | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 17 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 18 | Product Information. Xenical (orlistat). Roche Laboratories, Nutley, NJ. | ||||
| 19 | MacWalter RS, Fraser HW, Armstrong KM "Orlistat enhances warfarin effect." Ann Pharmacother 37 (2003): 510-2. [PMID: 12659605] | ||||
| 20 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 21 | MHRA. Medicines and Healthcare Products Regulatory Agency "Orlistat: theoretical interaction with antiretroviral HIV medicines.". | ||||
| 22 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 23 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 24 | Canadian Pharmacists Association. | ||||
| 25 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 26 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 27 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
