Details of the Drug
General Information of Drug (ID: DMW542E)
| Drug Name |
Orphenadrine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Antiflex; Biorphen; Brocadisipal; Brocasipal; Disipal; Lysantin; Mefenamine; Mephenamine; Methyldiphenylhydramine; Myolin; Myotrol; ORP; Orfenadrina; Orfro; Orphenadine; Orphenadinum; Orphenadrin; Orphenadrinum; Orphenate; Orphenedrine; Norflex Orphenadrine Citrate; Sodium Mefenamine; WS 2434; Banflex (TN); Biorphen (TN); Brocasipal (TN); Disipal (TN); Flexon (TN); Mephenamin (TN); Mialgin (TN); Norflex (TN); O-Methyldiphenhydramine; O-Monomethyldiphenhydramine; Orfenadrina [INN-Spanish]; Orphenadrine(INN); Orphenadrine [INN:BAN]; Orphenadrinum [INN-Latin]; Beta-Dimethylaminoethyl 2-methylbenzhydryl ether; Phenyl-o-tolylmethyl dimethyaminoethyl ether; Beta-Dimethylaminoethyl-2-methylbenzhydryl ether; N,N-Dimethyl-2-(alpha-2-tolylbenzoyloxy)ethylamin; N,N-Dimethyl-2-[(2-methylphenyl)(phenyl)methoxy]ethanamine; N,N-dimethyl-2-(alpha-2-tolylbenzoyloxy)ethylamine; N,N-dimethyl-2-[(2-methylphenyl)-phenylmethoxy]ethanamine; N,N-Dimethyl-2-(o-methyl-alpha-phenylbenzyl)oxy)ethylamine; N,N-Dimethyl-2-[(o-methyl-alpha-phenylbenzyl)oxy]ethylamine; N,N-dimethyl-2-{[(2-methylphenyl)(phenyl)methyl]oxy}ethanamine; 2-(Phenyl-o-tolylmethoxy)ethyldimethylamine; 2-Methyldiphenhydramine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antiparkinson Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
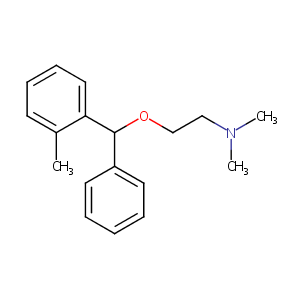 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 269.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Orphenadrine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7251). | ||||
|---|---|---|---|---|---|
| 2 | Orphenadrine FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Involvement of voltage-gated sodium channels blockade in the analgesic effects of orphenadrine. Pain. 2009 Apr;142(3):225-35. | ||||
| 7 | Examination of purported probes of human CYP2B6. Pharmacogenetics. 1997 Jun;7(3):165-79. | ||||
| 8 | Essential requirements for substrate binding affinity and selectivity toward human CYP2 family enzymes. Arch Biochem Biophys. 2003 Jan 1;409(1):32-44. | ||||
| 9 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 10 | Kulik AV, Wilbur R "Delirium and stereotypy from anticholinergic antiparkinson drugs." Prog Neuropsychopharmacol Biol Psychiatry 6 (1982): 75-82. [PMID: 7202232] | ||||
| 11 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 12 | Cole JM, Sheehan AH, Jordan JK "Concomitant use of ipratropium and tiotropium in chronic obstructive plmonary disease." Ann Pharmacother 46 (2012): 1717-21. [PMID: 23170031] | ||||
| 13 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 14 | Cohen MA, Alfonso CA, Mosquera M. Development of urinary retention during treatment with clozapine and meclizine [published correction appears in Am J Psychiatry 1994 Jun;151(6):952]. Am J Psychiatry. 1994;151(4):619-620. [PMID: 8147469] | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 17 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 18 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
