Details of the Drug
General Information of Drug (ID: DMY38SB)
| Drug Name |
CATECHIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cianidanol; (+)-catechin; 154-23-4; Catechuic acid; Cyanidanol; Catechinic acid; D-Catechin; Catergen; Cianidol; (+)-Cyanidanol; (+)-Cyanidan-3-ol; Biocatechin; (+)-Catechin Hydrate; D-(+)-Catechin; Dexcyanidanol; Catechin (flavan); Catechol (flavan); D-Catechol; (2R,3S)-Catechin; (2R,3S)-2-(3,4-Dihydroxyphenyl)chroman-3,5,7-triol; Gambier; 3-Cyanidanol, (+)-; Cianidanolum; Catechu; Transepar; Katha; (2R,3S)-(+)-Catechin; Cutch (dye); (+)-Cianidanol; 3,3',4',5,7-Flavanpentol; KB-53; (+)-Cyanidanol-3; CCRIS 6855
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
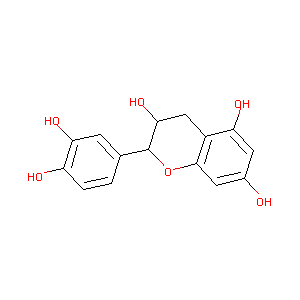 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 290.27 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
