Details of the Drug
General Information of Drug (ID: DMY64HE)
| Drug Name |
alpha-linolenic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Linolenic acid; linolenic acid; alpha-Linolenic acid; 463-40-1; linolenate; (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid; a-Linolenic acid; cis,cis,cis-9,12,15-Octadecatrienoic acid; all-cis-9,12,15-Octadecatrienoic acid; alpha-Linolenate; 9-cis,12-cis,15-cis-Octadecatrienoic acid; (Z,Z,Z)-9,12,15-Octadecatrienoic acid; Linolenic acid (8CI); 9,12,15-Octadecatrienoic acid, (Z,Z,Z)-; (9,12,15)-linolenic acid; alpha-Lnn; 9Z,12Z,15Z-Octadecatrienoic acid; Industrene 120; CCRIS 656; UNII-0RBV727H71; (9Z,12Z,15Z)-Octadecatrienoic acid; linolenate; alpha-LA; C18:3; LINOLENIC ACID
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
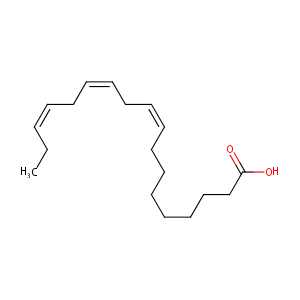 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 278.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
References
