Details of the Drug
General Information of Drug (ID: DMYLMU0)
| Drug Name |
Isosorbide Mononitrate
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Conpin; Corangin; Duride; Edistol; Elantan; Epicordin; Etimonis; IHD; ISMN; ISMO; Imazin; Imdur; Imodur; Imtrate; Ismexin; Ismox; Isomon; Isomonat; Isomonit; Iturol; Medocor; Monicor; Monis; Monisid; Monit; Monizid; MonoSigma; Monocedocard; Monocinque; Monoclair; Monocord; Monoket; Monolong; Monomax; Mononit; Monopront; Monosorb; Monosorbitrate; Monosordil; Monotrate; Multitab; Nitex; Nitramin; Olicard; Olicardin; Orasorbil; Percorina; Pertil; Plodin; Promocard; Sigacora; Sorbimon; Titarane; Turimonit; Uniket; Vasdilat; Vasotrate; Conpin Retardkaps; Corangin SR; Elantan Long; Elantan Retard; ISMN AL; ISMN AbZ; ISMN Apogepha; ISMN Atid; ISMN Basics; ISMN Heumann; ISMN Hexal; ISMN Lannacher; ISMN Stada; Imdur Durules; Isosorbidi mononitras; Isosorbidi mononitras [Latin]; Mono Corax; Mono Corax Retard; Mono Mack; Monodur Durules; Monoket OD; Monoket Retard; Mononitrato de isosorbida; Mononitrato de isosorbida [Spanish]; AHR 4698; IS 5MN; Imdur 60; Monit 20; Mono Mac 50D; Monocord 20; Monocord 40; Monocord 50 SR; Monolong 40; Monolong 60; Mononit 20; Mononit 40; Mononit Retard 50; Monosorb XL 60;Olicard 40; Pentacard 20; AHR-4698; BM 22-145; BM 22.145; Chemydur (TN); Fem-Mono; IS 5-MN; Imdur (TN); Ismo (TN); Ismo-20; Isopen-20; Isosorbide 5-mononitrate; Isosorbide 5-nitrate; Mono-Mack; Mono-Sanorania; Mononitrate d'isosorbide; Mononitrate d'isosorbide [French]; Olicard-retard; BM-22-145; Isosobide-5-mononitrate [UN3251] [Flammable solid]; Isosorbide-5-mononitrate; Isosorbide-5-nitrate; Isosorbide mononitrate (JAN/USP/INN); Isosorbide mononitrate [USAN:BAN:INN:JAN]; Isosorbide mononitrate [USAN:INN:BAN:JAN]; D-Glucitol, 1,4:3,6-dianhydro-, 5-nitrate; [(3S,3aR,6R,6aS)-3-hydroxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-6-yl] nitrate; 1,4:3,6-Dianhydro-D-glucitol 5-nitrate; 1,4:3,6-dianhydro-5-O-nitro-D-glucitol; 5-ISMN Durules; 5-Ismn
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Vasodilator Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
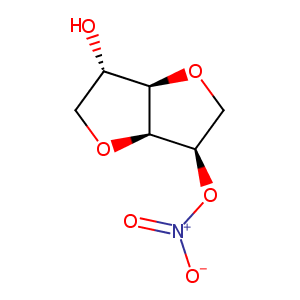 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 191.14 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Isosorbide Mononitrate (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Isosorbide mononitrate FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Clinical pharmacokinetics of mefloquine. Clin Pharmacokinet. 1990 Oct;19(4):264-79. doi: 10.2165/00003088-199019040-00002. | ||||
| 5 | Abshagen UW: Pharmacokinetics of isosorbide mononitrate. Am J Cardiol. 1992 Nov 27;70(17):61G-66G. doi: 10.1016/0002-9149(92)90028-w. | ||||
| 6 | FDA Approved Drug Products: Monoket (isosorbide mononitrate) tablets | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 9 | Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem. 2010 Jan;333(1-2):191-201. | ||||
| 10 | Isoforms of cytochrome P450 on organic nitrate-derived nitric oxide release in human heart vessels. FEBS Lett. 1999 Jun 11;452(3):165-9. | ||||
| 11 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 12 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 13 | Katz RJ, Levy WS, Buff L, Wasserman AG "Prevention of nitrate tolerance with angiotension converting enzyme inhibitors." Circulation 83 (1991): 1271-7. [PMID: 1901528] | ||||
| 14 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
