Details of the Drug
General Information of Drug (ID: DMOSW35)
| Drug Name |
Felodipine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Agon; Felobeta; Felocor; Feloday; Felodipina; Felodipinum; Felodur; Felogamma; Felogard; Fensel; Flodil; Hydac; Lexxel; Logimax; Modip; Munobal; Penedil; Perfudal; Plandil; Plendil; Preslow; Prevex; Renedil; Splendil; AGON SR; AbZ Brand of Felodipine; Aliud Brand of Felodipine; Alphapharm Brand of Felodipine; Alpharma Brand of Felodipine; Astra Brand of Felodipine; AstraZeneca Brand of Felodipine; Aventis Brand of Felodipine; Azupharma Brand of Felodipine; BC Brand of Felodipine; Betapharm Brand of Felodipine; Ct Arzneimittel Brand of Felodipine; Felo Biochemie; Felo Puren; Felodipin AL; Felodipin AZU; Felodipin AbZ; Felodipin Heumann; Felodipin Stada; Felodipin dura; Felodipin ratiopharm; Felodipin von ct; FelodurER; Heumann Brand of Felodipine; Hexal Brand of Felodipine; Hoechst Brand of Felodipine; Merck dura Brand of Felodipine; Munobal Retard; Pharmaceutica Astra Brand of Felodipine; Pharmacia Spain Brandof Felodipine; Plendil Depottab; Plendil ER; Plendil Retard; Promed Brand of Felodipine; Ratiopharm Brand of Felodipine; Stadapharm Brand of Felodipine; TheraPharm Brand of Felodipine; Worwag Brand of Felodipine; F 9677; Felodipin 1A Pharma; H 154 82; H 15482; CGH-869; Ct-Arzneimittel Brand of Felodipine; Dl-Felodipine; Felo-Puren; Felodipin-ratiopharm; Felodipina [INN-Spanish]; Felodipinum [INN-Latin]; H 154-82; H 154/82; Heumann, Felodipin; Plendil (TN); AE-641/11429675; Felodipine [USAN:BAN:INN]; Felodipine [USAN:INN:BAN]; H-154/82; Felodipine (JAN/USP/INN); Plendil, Renedil, Feloday, Felodipine; Ethyl methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; (+-)-Ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate; (+/-)-ethylmethyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate; 1A Brand of Felodipine; 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-4-(2,3-dichlorophenyl)-2,6-dimethyl-, ethyl methylester; 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-4-(2,3-dichlorophenyl)-2,6-dimethyl-,ethyl methyl ester; 3,5-Pyridinedicarboxylic acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, ethyl methyl ester; 3-Ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate; 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; 4-(2,3-Dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinecarboxylic acid ethyl methyl ester; 4-(2,3-dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid 3-ethyl ester 5-methyl ester; 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid ethyl methyl ester; 5-O-ethyl 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
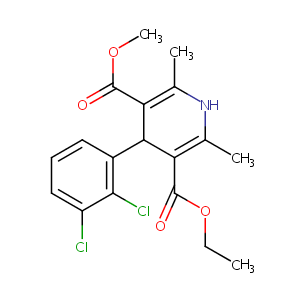 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 384.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Felodipine
Coadministration of a Drug Treating the Disease Different from Felodipine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4190). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. J Pharmacol Exp Ther. 2009 Feb;328(2):621-7. | ||||
| 6 | The influence of CYP3A5*3 and BCRPC421A genetic polymorphisms on the pharmacokinetics of felodipine in healthy Chinese volunteers. J Clin Pharm Ther. 2017 Jun;42(3):345-349. | ||||
| 7 | Drug interactions with calcium channel blockers: possible involvement of metabolite-intermediate complexation with CYP3A. Drug Metab Dispos. 2000 Feb;28(2):125-30. | ||||
| 8 | Drug Interactions Flockhart Table | ||||
| 9 | Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Sci Rep. 2014 Sep 26;4:6437. doi: 10.1038/srep06437. | ||||
| 10 | Optical isomers of dihydropyridine calcium channel blockers display enantiospecific effects on the expression and enzyme activities of human xenobiotics-metabolizing cytochromes P450. Toxicol Lett. 2016 Nov 16;262:173-186. | ||||
| 11 | A Dual Non-ATP Analogue Inhibitor of Aurora Kinases A and B, Derived from Resorcinol with a Mixed Mode of Inhibition. Chem Biol Drug Des. 2016 Jun;87(6):958-67. doi: 10.1111/cbdd.12728. Epub 2016 Mar 21. | ||||
| 12 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | ||||
| 13 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 14 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 15 | Canadian Pharmacists Association. | ||||
| 16 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 17 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 18 | Product Information. Mycobutin (rifabutin). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 19 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 20 | Heinig R, Adelmann HG, Ahr G "The effect of ketoconazole on the pharmacokinetics, pharmacodynamics and safety of nisoldipine." Eur J Clin Pharmacol 55 (1999): 57-60. [PMID: 10206086] | ||||
| 21 | Bratel T, Billing B, Dahlqvist R "Felodipine reduces the absorption of theophylline in man." Eur J Clin Pharmacol 36 (1989): 481-5. [PMID: 2753066] | ||||
| 22 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 23 | Bailey DG, Bend JR, Arnold JMO, Tran LT, Spence JD "Erythromycin-felodipine interaction: magnitude, mechanism, and comparison with grapefruit juice." Clin Pharmacol Ther 60 (1996): 25-33. [PMID: 8689808] | ||||
| 24 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 25 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 26 | Product Information. Talzenna (talazoparib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 27 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 28 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 29 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 30 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 31 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 32 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 33 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 34 | Bahls FH, Ozuna J, Ritchie DE "Interactions between calcium channel blockers and the anticonvulsants carbamazepine and phenytoin." Neurology 41 (1991): 740-2. [PMID: 2027492] | ||||
| 35 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 36 | Jalava KM, Olkkola KT, Neuvonen PJ "Itraconazole greatly increases plasma concentrations and effects of felodipine." Clin Pharmacol Ther 61 (1997): 410-5. [PMID: 9129558] | ||||
| 37 | Henry M, Kay MM, Viccellio P "Cardiogenic shock associated with calcium-channel and beta blockers: reversal with intravenous calcium chloride." Am J Emerg Med 3 (1985): 334-6. [PMID: 2860911] | ||||
| 38 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 39 | Product Information. Olysio (simeprevir). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 40 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 41 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 42 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 43 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 44 | Product Information. Reyataz (atazanavir). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 45 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 46 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 47 | Product Information. Movantik (naloxegol). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 48 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 49 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 50 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 51 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 52 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 53 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 54 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 55 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 56 | Product Information. Istodax (romidepsin). Gloucester Pharmaceuticals, Cambridge, MA. | ||||
| 57 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 58 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 59 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 60 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 61 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 62 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 63 | Product Information. Rapaflo (silodosin). Watson Pharmaceuticals, Corona, CA. | ||||
| 64 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 65 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 66 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 67 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 68 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
