Details of the Drug
General Information of Drug (ID: DMSYRBX)
| Drug Name |
Atazanavir
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
198904-31-3; Latazanavir; Zrivada; Reyataz; BMS-232632; BMS 232632; Atazanavir [INN:BAN]; CGP 73547; Atazanavir Base; UNII-QZU4H47A3S; CGP-73547; HSDB 7339; Reyataz (TN); ATV; QZU4H47A3S; CHEMBL1163; CHEBI:37924; (3S,8S,9S,12S)-3,12-BIS(1,1-DIMETHYLETHYL)-8-HYDROXY-4,11-DIOXO-9-(PHENYLMETHYL)-6-[[4-(2-PYRIDINYL)PHENYL]METHYL]-2,5,6,10,13-PENTAAZATETRADECANEDIOIC ACID DIMETHYL ESTER; NCGC00182552-01; AK174307; DSSTox_CID_28617; DSSTox_RID_82887; DSSTox_GSID_48691; DR7; atazanavirum; ATZ; Atazanavirum; Atazanavir (INN); Reyataz, BMS-232632, Atazanavir; Reyataz(TM) (*1:1 sulfate*); Dimethyl (3S,8S,9S,12S)-9-benzyl-3,12-di-tert-butyl-8-hydroxy-4,11-dioxo-6-[4-(2-pyridyl)benzyl]-2,5,6,10,13-pentaazatetradecanedioate; METHYL [(1S,4S,5S,10S)-4-BENZYL-1,10-DI-TERT-BUTYL-5-HYDROXY-2,9,12-TRIOXO-7-(4-PYRIDIN-2-YLBENZYL)-13-OXA-3,7,8,11-TETRAAZATETRADEC-1-YL]CARBAMATE; Methyl N-[(2S)-1-[2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-4-phenylbutyl]-2-[(4-pyridin-2-ylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate; (2S)-N-(3-{[(2S)-2-(Methoxycarbonylamino)-3,3-dimethylbutanoylamino][(4-(2-pyridyl)phenyl)methyl]amino}(1S,2S)-2-hydroxy-1-benzylpropyl)-2-(methoxycarbonylamino)-3,3-dimethylbutanamide
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
|||||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency Virus
|
|||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||
| Structure |
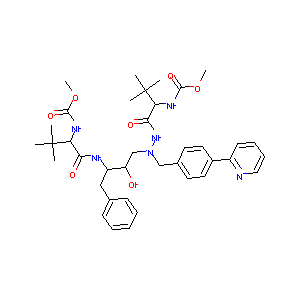 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 704.9 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.6 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 18 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | |||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Atazanavir
Coadministration of a Drug Treating the Disease Different from Atazanavir (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clin Pharmacol Ther. 2016 Apr;99(4):363-9. doi: 10.1002/cpt.269. Epub 2015 Nov 9. | ||||
| 7 | Hollow-fiber unit evaluation of a new human immunodeficiency virus type 1 protease inhibitor, BMS-232632, for determination of the linked pharmacod... J Infect Dis. 2001 Apr 1;183(7):1126-9. | ||||
| 8 | Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005 Jun;33(6):764-70. | ||||
| 9 | Atazanavir for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2004 Dec;24(12):1732-47. | ||||
| 10 | In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005 Nov;33(11):1729-39. | ||||
| 11 | Robustness testing and optimization of an adverse outcome pathway on cholestatic liver injury. Arch Toxicol. 2020 Apr;94(4):1151-1172. doi: 10.1007/s00204-020-02691-9. Epub 2020 Mar 10. | ||||
| 12 | Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286. | ||||
| 13 | Interactions of protease inhibitors atazanavir and ritonavir with ABCB1, ABCG2, and ABCC2 transporters: Effect on transplacental disposition in rats. Reprod Toxicol. 2018 Aug;79:57-65. doi: 10.1016/j.reprotox.2018.05.008. Epub 2018 May 30. | ||||
| 14 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | ||||
| 15 | Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12(4):489-500. | ||||
| 16 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 17 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 18 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Selzentry (maraviroc). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 21 | Product Information. Isentress (raltegravir). Merck & Company Inc, West Point, PA. | ||||
| 22 | Product Information. Reyataz (atazanavir). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 23 | Product Information. Cleocin (clindamycin). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 24 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 25 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 26 | Acosta EP, Henry K, Baken L, Page LM, Fletcher CV "Indinavir concentrations and antiviral effect." Pharmacotherapy 19 (1999): 708-12. [PMID: 10391416] | ||||
| 27 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 28 | Product Information. Olinvyk (oliceridine). Trevena Inc, Chesterbrook, PA. | ||||
| 29 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 30 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 31 | Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN "Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole." Ann Pharmacother 38 (2004): 46-9. [PMID: 14742792] | ||||
| 32 | Product Information. Vraylar (cariprazine). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 33 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 34 | Product Information. Ixempra (ixabepilone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 35 | Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D "Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects." Br J Clin Pharmacol 71 (2011): 522-7. [PMID: 21395644] | ||||
| 36 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 37 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 38 | Product Information. Ibrance (palbociclib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 39 | Barry M, Mulcahy F, Merry C, Gibbons S, Back D "Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection." Clin Pharmacokinet 36 (1999): 289-304. [PMID: 10320951] | ||||
| 40 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 41 | Product Information. Breo Ellipta (fluticasone-vilanterol). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 42 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 43 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 44 | Product Information. Fetzima (levomilnacipran). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 45 | Hermans JJ, Thijssen HH "Human liver microsomal metabolism of the enantiomers of warfarin and acenocoumarol: P450 isozyme diversity determines the differences in their pharmacokinetics." Br J Pharmacol 110 (1993): 482-90. [PMID: 8220911] | ||||
| 46 | Bailey DG, Arnold JMO, Spence JD "Grapefruit juice and drugs - how significant is the interaction." Clin Pharmacokinet 26 (1994): 91-8. [PMID: 8162660] | ||||
| 47 | Canadian Pharmacists Association. | ||||
| 48 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 49 | Product Information. Viibryd (vilazodone). Trovis Pharmaceuticals LLC, New Haven, CT. | ||||
| 50 | Product Information. Celexa (citalopram). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 51 | Product Information. Rexulti (brexpiprazole). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 52 | Eap CB, Bender S, Gastpar M, et al "Steady state plasma levels of the enantiomers of trimipramine and its metabolites in CYP2D6-, CYP2C19- and CYP3A4/5-phenotyped patients." Ther Drug Monit 22 (2000): 209-14. [PMID: 10774635] | ||||
| 53 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 54 | Product Information. Osphena (ospemifene). Shionogi USA Inc, Florham Park, NJ. | ||||
| 55 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 56 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 57 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 58 | Akdag I, Ersoy A, Kahvecioglu S, Gullulu M, Dilek K "Acute colchicine intoxication during clarithromycin administration in patients with chronic renal failure." J Nephrol 19 (2006): 515-7. [PMID: 17048210] | ||||
| 59 | Product Information. Norvir (ritonavir). Abbott Pharmaceutical, Abbott Park, IL. | ||||
| 60 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 61 | Product Information. Xerava (eravacycline). Tetraphase Pharmaceuticals, Inc, Watertown, MA. | ||||
| 62 | Product Information. Belsomra (suvorexant). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 63 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 64 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 65 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 66 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 67 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 68 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 69 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 70 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 71 | Abernethy DR, Wesche DL, Barbey JT, et al. "Stereoselective halofantrine disposition and effect: concentration-related QTc prolongation." Br J Clin Pharmacol 51 (2001): 231-7. [PMID: 11298069] | ||||
| 72 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 73 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 74 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 75 | Product Information. Imbruvica (ibrutinib). Pharmacyclics Inc, Sunnyvale, CA. | ||||
| 76 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 77 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 78 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 79 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 80 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 81 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 82 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 83 | Hamberg P, Woo MM, Chen LC, et al. "Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor." Cancer Chemother Pharmacol 68 (2011): 805-13. [PMID: 21706316] | ||||
| 84 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 85 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 86 | Product Information. Jakafi (ruxolitinib). Incyte Corporation, Wilmington, DE. | ||||
| 87 | Product Information. Akynzeo (netupitant-palonosetron). Eisai Inc, Woodcliff Lake, NJ. | ||||
| 88 | MHRA. Medicines and Healthcare Products Regulatory Agency "Orlistat: theoretical interaction with antiretroviral HIV medicines.". | ||||
| 89 | Product Information. Orlaam (levomethadyl acetate) Roxanne Laboratories Inc, Columbus, OH. | ||||
| 90 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 91 | Product Information. Nourianz (istradefylline). Kyowa Kirin, Inc, Bedminster, NJ. | ||||
| 92 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 93 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 94 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 95 | Baldwin ZK, Ceraldi CC "Ergotism associated with HIV antiviral protease inhibitor therapy." J Vasc Surg 37 (2003): 676-8. [PMID: 12618710] | ||||
| 96 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 97 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
| 98 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 99 | Product Information. Letairis (ambrisentan). Gilead Sciences, Foster City, CA. | ||||
| 100 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 101 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 102 | DeVane CL, Nemeroff CB "Clinical pharmacokinetics of quetiapine - An atypical antipsychotic." Clin Pharmacokinet 40 (2001): 509-22. [PMID: 11510628] | ||||
| 103 | Product Information. Abilify (aripiprazole). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 104 | Product Information. Fanapt (iloperidone). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 105 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 106 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 107 | Product Information. Beleodaq (belinostat). Spectrum Pharmaceuticals Inc, Irvine, CA. | ||||
| 108 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 109 | Barry M, Gibbons S, Back D, Mulcahy F "Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations." Clin Pharmacokinet 32 (1997): 194-209. [PMID: 9084959] | ||||
| 110 | Bundow D, Aboulafia DM "Potential drug interaction with paclitaxel and highly active antiretroviral therapy in two patients with AIDS-associated Kaposi sarcoma." Am J Clin Oncol 27 (2004): 81-4. [PMID: 14758138] | ||||
| 111 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 112 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
| 113 | Arrington-Sanders R, Hutton N, Siberry GK "Ritonavir-fluticasone interaction causing Cushing syndrome in HIV-infected children and adolescents." Pediatr Infect Dis J 25 (2006): 1044-1048. [PMID: 17072128] | ||||
| 114 | Gelosa P, Castiglioni L, Tenconi M, et.al "Pharmacokinetic drug interactions of the non-vitamin K antagonist oral anticoagulants (NOACs)" Pharmacol Res 135 (2018): 60-79. [PMID: 30040996] | ||||
