| 1 |
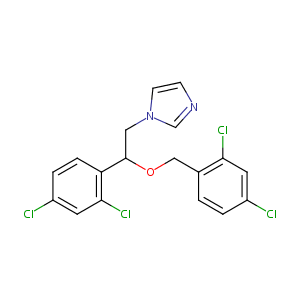
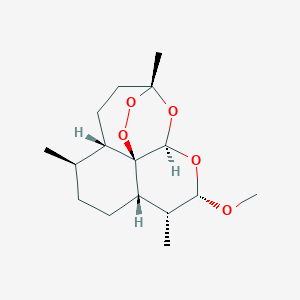
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
Miconazole FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2449).
|
| 4 |
The fight against drug-resistant malaria: novel plasmodial targets and antimalarial drugs. Curr Med Chem. 2008;15(2):161-71.
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 6 |
Inhibition of cytochrome P450 enzymes participating in p-nitrophenol hydroxylation by drugs known as CYP2E1 inhibitors. Chem Biol Interact. 2004 Apr 15;147(3):331-40.
|
| 7 |
Azole antimycotics differentially affect rifampicin-induced pregnane X receptor-mediated CYP3A4 gene expression. Drug Metab Dispos. 2008 Feb;36(2):339-48.
|
| 8 |
The novel azole R126638 is a selective inhibitor of ergosterol synthesis in Candida albicans, Trichophyton spp., and Microsporum canis. Antimicrob Agents Chemother. 2004 Sep;48(9):3272-8.
|
| 9 |
Comparative assessment of the inhibition of recombinant human CYP19 (aromatase) by azoles used in agriculture and as drugs for humans. Endocr Res. 2004 Aug;30(3):387-94.
|
| 10 |
Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol Pharmacol. 2006 Jul;70(1):329-39.
|
| 11 |
In vitro inhibitory effects of asiaticoside and madecassoside on human cytochrome P450. Toxicol In Vitro. 2011 Jun;25(4):890-6.
|
| 12 |
Direct stimulation of adenylyl cyclase 9 by the fungicide imidazole miconazole. Naunyn Schmiedebergs Arch Pharmacol. 2019 Apr;392(4):497-504. doi: 10.1007/s00210-018-01610-1. Epub 2019 Jan 3.
|
| 13 |
The classic azole antifungal drugs are highly potent endocrine disruptors in vitro inhibiting steroidogenic CYP enzymes at concentrations lower than therapeutic Cmax. Toxicology. 2019 Sep 1;425:152247. doi: 10.1016/j.tox.2019.152247. Epub 2019 Jul 19.
|
| 14 |
Anti-mycotics suppress interleukin-4 and interleukin-5 production in anti-CD3 plus anti-CD28-stimulated T cells from patients with atopic dermatitis. J Invest Dermatol. 2001 Dec;117(6):1635-46. doi: 10.1046/j.0022-202x.2001.01566.x.
|
| 15 |
Miconazole induces protective autophagy in bladder cancer cells. Environ Toxicol. 2021 Feb;36(2):185-193. doi: 10.1002/tox.23024. Epub 2020 Sep 27.
|
| 16 |
Why are most phospholipidosis inducers also hERG blockers?. Arch Toxicol. 2017 Dec;91(12):3885-3895. doi: 10.1007/s00204-017-1995-9. Epub 2017 May 27.
|
| 17 |
Differential azole antifungal efficacies contrasted using a Saccharomyces cerevisiae strain humanized for sterol 14 alpha-demethylase at the homolo... Antimicrob Agents Chemother. 2008 Oct;52(10):3597-603.
|
| 18 |
The effect of grapefruit juice on the time-dependent decline of artemether plasma levels in healthy subjects. Clin Pharmacol Ther. 1999 Oct;66(4):408-14.
|
| 19 |
The contribution of the enzymes CYP2D6 and CYP2C19 in the demethylation of artemether in healthy subjects. Eur J Drug Metab Pharmacokinet. 1998 Jul-Sep;23(3):429-36.
|
| 20 |
Effect of pharmacogenetics on plasma lumefantrine pharmacokinetics and malaria treatment outcome in pregnant women. Malar J. 2017 Jul 3;16(1):267.
|
| 21 |
Pharmacokinetic interaction between etravirine or darunavir/ritonavir and artemether/lumefantrine in healthy volunteers: a two-panel, two-way, two-period, randomized trial. HIV Med. 2013 Aug;14(7):421-9.
|
| 22 |
Insights into CYP2B6-mediated drug-drug interactions. Acta Pharm Sin B. 2016 Sep;6(5):413-425.
|
| 23 |
Fetal bovine serum and human constitutive androstane receptor: evidence for activation of the SV23 splice variant by artemisinin, artemether, and arteether in a serum-free cell culture system. Toxicol Appl Pharmacol. 2014 Jun 1;277(2):221-30. doi: 10.1016/j.taap.2014.03.023. Epub 2014 Apr 12.
|
|
|
|
|
|
|