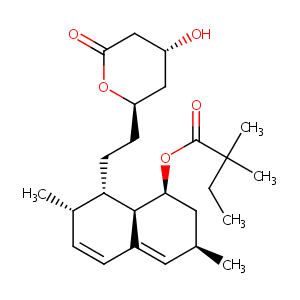
| Synonyms |
Cholestat; Coledis; Colemin; Corolin; Denan; Labistatin; Lipex; Lipovas; Lodales; Medipo; Nivelipol; Pantok; Rendapid; Simovil; Simvastatina; Simvastatine; Simvastatinum; Sinvacor; Sivastin; Synvinolin; Vasotenal; Zocor; Zocord; Simvast CR; Simvastatina [Spanish]; Simvastatine [French]; Simvastatinum [Latin]; MK 0733; MK 733; MK733; TNP00259; DRG-0320; KS-1113; L 644128-000U; MK-0733; MK-733; Simcard (TN); Simlup (TN); Simvacor (TN); Simvastatin & Primycin; Simvastatin, Compactin; Zocor (TN); Simvastatin [USAN:INN:BAN]; Simvastatin (JAN/USP/INN); Zocor, Simlup, Simcard, Simvacor, Simvoget, Zorced, Simvastatin; [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,*aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester; (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate; Butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1 alpha,3 alpha,7 beta,8 beta(2S*,4S*),-8a beta; 2,2-Dimethylbutanoic acid (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester; 2,2-Dimethylbutyric acid, 8-ester with (4R,6R)-6-(2-((1S,2S,6R,8S,8aR)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2H-pyran-2-one
|