Details of the Drug
General Information of Drug (ID: DM0N8ZM)
| Drug Name |
COUMARIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
coumarin; 2H-Chromen-2-one; 91-64-5; 2H-1-Benzopyran-2-one; cumarin; 1,2-Benzopyrone; Rattex; Tonka bean camphor; Coumarinic anhydride; chromen-2-one; Coumarine; Benzo-alpha-pyrone; cis-o-Coumarinic acid lactone; o-Hydroxycinnamic acid lactone; o-Hydroxycinnamic lactone; 2-Oxo-1,2-benzopyran; Coumarinic lactone; Kumarin; Benzo-a-pyrone; Kumarin [Czech]; 5,6-Benzo-2-pyrone; 2H-1-Benzopyran, 2-oxo-; 5,6-Benzo-alpha-pyrone; 2H-Benzo(b)pyran-2-one; 2H-Benzo[b]pyran-2-one; Caswell No. 259C; o-Coumaric acid lactone
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
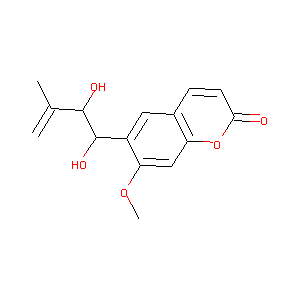 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 146.14 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
