Details of the Drug
General Information of Drug (ID: DM46F5X)
| Drug Name |
isobutylmethylxanthine
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3-Isobutyl-1-methylxanthine; IBMX; 28822-58-4; 1-METHYL-3-ISOBUTYLXANTHINE; Methylisobutylxanthine; 3-Isobutyl-1-methyl-1H-purine-2,6(3H,7H)-dione; Xanthine, 3-isobutyl-1-methyl-; 3-Isobutyl-1-methyxanthine; 1H-Purine-2,6-dione, 3,7-dihydro-1-methyl-3-(2-methylpropyl)-; Methyl-isobutylxanthine; 3-isobutyl-1-methylxanthine (ibmx); UNII-TBT296U68M; 3-Isobutyl 1-methylxanthine; NSC 165960; CCRIS 4290; CHEBI:34795; 1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione; 3-isobutyl-1-methyl-7H-xanthine; methylisobutylxanthine; 3-Isobutyl-1-methyl-3,9-dihydro-purine-2,6-dione; 3-ISOBUTYL-1-METHYLXANTHINE
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||
| Structure |
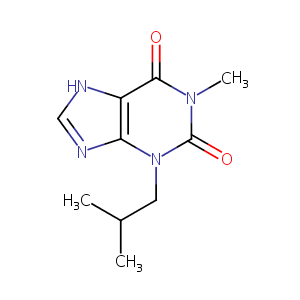 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 222.24 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
