Details of the Drug
General Information of Drug (ID: DM4ES8F)
| Drug Name |
Penbutolol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Betapressin; Levatol; Levatolol; Levopenbutol; Lobeta; Paginol; Penbutololum; PENBUTOLOL SULFATE; HOE 893; HOE 893d; Betapressin (TN); Hostabloc (TN); L-Penbutolol; Levatol (TN); Levatolol (TN); Lobeta (TN); Paginol (TN); Penbutolol (INN); Penbutolol [INN:BAN]; Penbutololum [INN-Latin]; Penbutolol Sulfate (2:1); S(-)-Penbutolol; (-)-Penbutolol; (2S)-1-(tert-butylamino)-3-(2-cyclopentylphenoxy)propan-2-ol; 1-(tert-Butylamino)-3-(o-cyclopentylphenoxy)propan-2-ol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
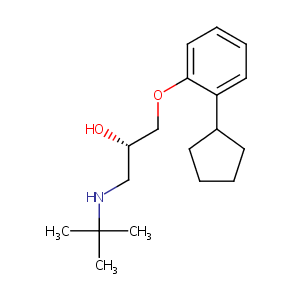 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 291.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Penbutolol
Coadministration of a Drug Treating the Disease Different from Penbutolol (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7263). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | beta-Adrenergic receptor blockers--a group of chiral drugs: different effects of each enantiomer. Ceska Slov Farm. 2002 May;51(3):121-8. | ||||
| 7 | Application of substrate depletion assay to evaluation of CYP isoforms responsible for stereoselective metabolism of carvedilol. Drug Metab Pharmacokinet. 2016 Dec;31(6):425-432. | ||||
| 8 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 9 | Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4. | ||||
| 10 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 11 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 12 | Chapple DJ, Clark JS, Hughes R "Interaction between atracurium and drugs used in anaesthesia." Br J Anaesth 55 Suppl 1 (1983): s17-22. [PMID: 6688011] | ||||
| 13 | Canadian Pharmacists Association. | ||||
| 14 | Briant RH, Dorrington RE, Ferry DG, Paxton JW "Bioavailability of metoprolol in young adults and the elderly, with additional studies on the effects of metoclopramide and probanthine." Eur J Clin Pharmacol 25 (1983): 353-6. [PMID: 6628522] | ||||
| 15 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 16 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 17 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 18 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 19 | Chrysant SG "Experience with terazosin administered in combination with other antihypertensive agents." Am J Med 80 (1986): 55-61. [PMID: 2872808] | ||||
