Details of the Drug
General Information of Drug (ID: DM6T924)
| Drug Name |
Gabapentin
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aclonium; GBN; Gabapen; Gabapentina; Gabapentine; Gabapentinium; Gabapentino; Gabapentinum; Gabapetin; Neurontin; Serada; Vultin; Apotex brand of gabapentin; Aventis brand of gabapentin; Gabapentin GR; Gabapentin Hexal; Gabapentin Stada; Gabapentino [Spanish]; Hexal brand of gabapentin; Novopharm brand of gabapentin; Parke Davis brand of gabapentin; Pfizer brand of gabapentin; Pharmascience brand of gabapentin; Ratiopharm brand of gabapentin; Stadapharm brand of gabapentin; GOE 2450; Go 3450; Apo-Gabapentin; DDS-2003; DM-1796; DM-5689; G-154; Gabapentin (Neurontin); Gabapentin-ratiopharm; Gabapentine [INN-French]; Gabapentino [INN-Spanish]; Gabapentinum [INN-Latin]; Goe-3450; Neurontin (TN); Novo-Gabapentin; PMS-Gabapentin; Warner-Lambert brand of gabapentin; Gabapentin [USAN:INN:BAN]; [1-(AMINOMETHYL)CYCLOHEXYL]ACETIC ACID; Gabapentin (JAN/USAN/INN); 1-(Aminomethyl)-cyclohexaneacetic acid; 1-(Aminomethyl)cyclohexaneacetic acid; 2-[1-(aminomethyl)cyclohexyl]acetic acid
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
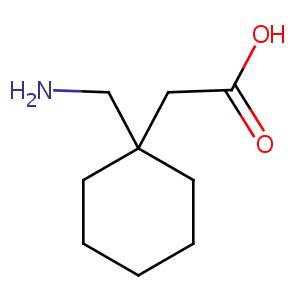 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 171.24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Gabapentin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5483). | ||||
|---|---|---|---|---|---|
| 2 | Gabapentin FDA Label | ||||
| 3 | FDA Approved Drug Products: CAMPRAL (acamprosate calcium) delayed-release tablets | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P: A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010 Oct;49(10):661-9. doi: 10.2165/11536200-000000000-00000. | ||||
| 7 | FDA Approved Drug Products: Neurontin (gabapentin) for oral use | ||||
| 8 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 9 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 10 | Gabapentin increases a tonic inhibitory conductance in hippocampal pyramidal neurons. Anesthesiology. 2006 Aug;105(2):325-33. | ||||
| 11 | Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol. 2013 Jun 1;85(11):1672-83. | ||||
| 12 | Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther. 2008 Mar;83(3):416-21. | ||||
| 13 | Clinical pharmacokinetic drug interaction studies of gabapentin enacarbil, a novel transported prodrug of gabapentin, with naproxen and cimetidine. Br J Clin Pharmacol. 2010 May;69(5):498-507. | ||||
| 14 | Functional evaluation of polymorphisms in the human ABCB1 gene and the impact on clinical responses of antiepileptic drugs. Pharmacogenet Genomics. 2008 May;18(5):390-402. doi: 10.1097/FPC.0b013e3282f85e36. | ||||
| 15 | Establishment of a 13 genes-based molecular prediction score model to discriminate the neurotoxic potential of food relevant-chemicals. Toxicol Lett. 2022 Feb 1;355:1-18. doi: 10.1016/j.toxlet.2021.10.013. Epub 2021 Nov 5. | ||||
| 16 | Eipe N, Penning J "Postoperative respiratory depression associated with pregabalin: a case series and a preoperative decision algorithm." Pain Res Manag 16 (2011): 353-6. [PMID: 22059207] | ||||
| 17 | Belcastro V, Costa C, Striano P "Levetiracetam-associated hyponatremia." Seizure 17 (2008): 389-90. [PMID: 18584781] | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 20 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 21 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 22 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 23 | Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G "Gabapentin enhances the analgesic effect of morphine in healthy volunteers." Anesth Analg 91 (2000): 185-91. [PMID: 10866910] | ||||
