Details of the Drug
General Information of Drug (ID: DM8EU0Z)
| Drug Name |
Dicloxacillin
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dichloroxacillin; Diclossacillina; Dicloxaciclin; Dicloxacilin; Dicloxacilina; Dicloxacilline; Dicloxacillinum; Dicloxacycline; Dycill; Dynapen; Maclicine; Methyldichlorophenylisoxazolylpenicillin; Pathocil; Diclossacillina [DCIT]; Dicloxacillin sodium; BRL 1702; Diclocil (TN); Dicloxacilina [INN-Spanish]; Dicloxacilline [INN-French]; Dicloxacillinum [INN-Latin]; R-13423; Dicloxacillin (USAN/INN); Dicloxacillin [USAN:INN:BAN]; Dicloxacillin, Monosodium Salt, Mono-Hydrate; (2S,5R,6R)-6-({[3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-({[3-(2,6-dichlorophenyl)-5-methylisoxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-[[3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolylpenicillin; 6-(3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolecarboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid; 6-(3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolecarboxamido)penicillanic acid; 6beta-{[3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazol-4-yl]carboxamido}-2,2-dimethylpenam-3alpha-carboxylic acid
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
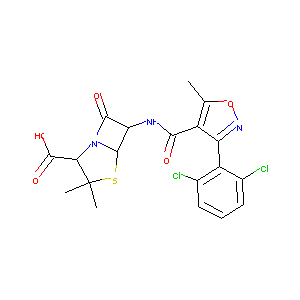 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 470.3 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Dicloxacillin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 062286. | ||||
|---|---|---|---|---|---|
| 2 | Dicloxacillin FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Mechanisms of resistance to beta-lactam antibiotics in Staphylococcus aureus. Scand J Infect Dis Suppl. 1984;42:64-71. | ||||
| 8 | Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60. | ||||
| 9 | Can the enhanced renal clearance of antibiotics in cystic fibrosis patients be explained by P-glycoprotein transport? Pharm Res. 2002 Apr;19(4):457-62. | ||||
| 10 | Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500. | ||||
| 11 | A comprehensive in vitro and in silico analysis of antibiotics that activate pregnane X receptor and induce CYP3A4 in liver and intestine. Drug Metab Dispos. 2008 Aug;36(8):1689-97. | ||||
| 12 | Dicloxacillin and erythromycin at high concentrations increase ICAM-1 expression by endothelial cells: a possible factor in the pathogenesis of infusion phlebitis. J Antimicrob Chemother. 2004 Feb;53(2):174-9. doi: 10.1093/jac/dkh056. Epub 2004 Jan 16. | ||||
| 13 | Screening of a chemical library reveals novel PXR-activating pharmacologic compounds. Toxicol Lett. 2015 Jan 5;232(1):193-202. doi: 10.1016/j.toxlet.2014.10.009. Epub 2014 Oct 16. | ||||
| 14 | Prediction of drug-induced liver injury using keratinocytes. J Appl Toxicol. 2017 Jul;37(7):863-872. doi: 10.1002/jat.3435. Epub 2017 Jan 31. | ||||
| 15 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 16 | Allen MB, Fitzpatrick RW, Barratt A, Cole RB "The use of probenecid to increase the serum amoxycillin levels in patients with bronchiectasis." Respir Med 84 (1990): 143-6. [PMID: 2371437] | ||||
| 17 | Kwon OC, Lee JS, Kim YG, Lee CK, Yoo B, Hong S. Safety of the concomitant use of methotrexate and a prophylactic dose of trimethoprim-sulfamethoxazole.?Clin Rheumatol. 2018;37(12):3215-3220. [PMID: 29383453] | ||||
