Details of the Drug
General Information of Drug (ID: DM8XP26)
| Drug Name |
Rolapitant
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Rolapitant; 552292-08-7; Sch 619734; Varubi; UNII-NLE429IZUC; SCH-619734; NLE429IZUC; 914462-92-3; (5S,8S)-8-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)methyl)-8-phenyl-1,7-diazaspiro[4.5]decan-2-one; (5S,8S)-8-[[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]-8-phenyl-1,9-diazaspiro[4.5]decan-2-one; Rolapitant [USAN:INN]; Varuby; Varubi (TN); Rolapitant (USAN/INN); Rolapitant(sch619734); SCHEMBL354305; GTPL5749; CHEMBL3707331; CHEBI:90908; DTXSID90203740; MolPort-035-942-975; EX-A1288; BCP09609; ZINC3816514
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
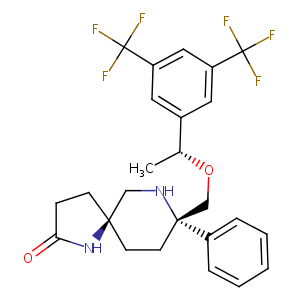 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 500.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chemotherapy-induced nausea | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | MD90 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Rolapitant (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5749). | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Netupitant and palonosetron trigger NK1 receptor internalization in NG108-15 cells. Exp Brain Res. 2014 Aug;232(8):2637-44. | ||||
| 4 | Pharmacokinetic interactions of rolapitant with cytochrome P450 3A substrates in healthy subjects. J Clin Pharmacol. 2019 Apr;59(4):488-499. | ||||
| 5 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 6 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 7 | Product Information. Olinvyk (oliceridine). Trevena Inc, Chesterbrook, PA. | ||||
| 8 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 9 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 10 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 11 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 12 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 13 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 14 | Product Information. Orgovyx (relugolix). Myovant Sciences, Inc., Brisbane, CA. | ||||
| 15 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
