Details of the Drug
General Information of Drug (ID: DMD8Q3J)
| Drug Name |
Gemfibrozil
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ApoGemfibrozil; Ausgem; Bolutol; Brozil; Cholespid; Clearol; Decrelip; Elmogan; Fetinor; Fibratol; Fibrocit; GFZ; Gemd; Gemfibril; Gemfibromax; Gemfibrosil; Gemfibrozilo; Gemfibrozilum; Gemhexal; Gemlipid; Gemnpid; GenGemfibrozil; Genlip; Gozid; Hidil; Hipolixan; Innogem; Innogen; Ipolipid; Jezil; Lanaterom; Lifibron; Lipazil; Lipigem; Lipira; Lipizyl; Lipozid; Lipur; Litarek; Lopid; Micolip; Normolip; NuGemfibrozil; Pilder; Polyxit; Progemzal; Reducel; Regulip; Renabrazin; Sinelip; Synbrozil; Taborcil; Tentroc; Trialmin; Alphapharm Brand of Gemfibrozil; Apo Gemfibrozil; Apotex Brand of Gemfibrozil; Bayvit Brand of Gemfibrozil; Bexal Brand of Gemfibrozil; Biochemie Brand of Gemfibrozil; Bull Brand of Gemfibrozil; Cantabria Brand of Gemfibrozil; Chem mart Brand of Gemfibrozil; Chem mart Gemfibrozil; DBL Gemfibrozil; Douglas Brand of Gemfibrozil; Farmasierra Brand of Gemfibrozil; Faulding Brand of Gemfibrozil;Ferrer Brand of Gemfibrozil; Gemfibrozilo Bayvit; Gemfibrozilo Bexal; Gemfibrozilo Ur; Gen Gemfibrozil; GenRX Gemfibrozil; Genpharm Brand of Gemfibrozil; Gevilon Uno; Healthsense Brand of Gemfibrozil; Healthsense Gemfibrozil; Hexal Brand of Gemfibrozil; Ipsen Brand of Gemfibrozil; Lipox Gemfi; Lopid R; Menarini Brand of Gemfibrozil; Novo Gemfibrozil; Novopharm Brand of Gemfibrozil; Nu Gemfibrozil; Nu Pharm Brand of Gemfibrozil; PMS Gemfibrozil; Parke Davis Brand of Gemfibrozil; Pfizer Brand of Gemfibrozil; Pharmascience Brand of Gemfibrozil; Quimifar Brand of Gemfibrozil; SBPA Gemfibrozil; Sigma Brand of Gemfibrozil; TAD Brand of Gemfibrozil; Terry White Chemists Brand of Gemfibrozil; Terry White Chemists Gemfibrozil; United Drug Brand of Gemfibrozil; Warner Lambert Brand of Gemfibrozil; CI719; Gemfi 1A Pharma; Apo-Gemfibrozil; Bayvit, Gemfibrozilo; Gem-S; Gemfibrozil, GenRX; Gemfibrozil, Healthsense; Gemfibrozil, SBPA; Gemfibrozilo [INN-Spanish]; Gemfibrozilum [INN-Latin]; Gen-Fibro;Gen-Gemfibrozil; Jezil (TN); Lopid (TN); Low-Lip; Novo-Gemfibrozil; Nu-Gemfibrozil; Nu-Pharm Brand of Gemfibrozil; PMS-Gemfibrozil; TEVA-A; WL-Gemfibrozil; Warner-Lambert Brand of Gemfibrozil; GEMFIBROZIL (SEE ALSO PEROXISOME PROJECT (GEMFIBROZIL)); Gemfibrozil [USAN:BAN:INN]; Gen-Fibro (TN); PEROXISOME PROJECT (GEMFIBROZIL) (SEE ALSO GEMFIBROZIL); Gemfibrozil (JAN/USP/INN); Gemcor, Lopid, Jezil,Gen-Fibro, Gemfibrozil; 1A Brand of Gemfibrozil; 2,2-Dimethyl-5-(2,5-dimethylphenoxy)pentanoic acid; 2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaeure; 2,2-Dimethyl-5-(2,5-xylyloxy)valeriansaeure; 2,2-Dimethyl-5-(2,5-xylyloxy)valeric acid; 5-(2,5-Dimethyl-Phenoxy)-2,2-Dimethyl-Pentanoic Acid; 5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic acid; 5-[(2,5-dimethylphenyl)oxy]-2,2-dimethylpentanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antilipemic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
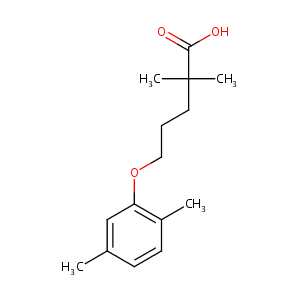 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 250.33 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Gemfibrozil (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3439). | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products: Gemfibrozil Oral Tablets | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Varma MV, Lin J, Bi YA, Kimoto E, Rodrigues AD: Quantitative Rationalization of Gemfibrozil Drug Interactions: Consideration of Transporters-Enzyme Interplay and the Role of Circulating Metabolite Gemfibrozil 1-O-beta-Glucuronide. Drug Metab Dispos. 2015 Jul;43(7):1108-18. doi: 10.1124/dmd.115.064303. Epub 2015 May 4. | ||||
| 6 | Farlow MR: Clinical pharmacokinetics of galantamine. Clin Pharmacokinet. 2003;42(15):1383-92. doi: 10.2165/00003088-200342150-00005. | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc). 2006 Jan;42(1):39-64. | ||||
| 9 | Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmacokinet. 1998 Feb;34(2):155-62. | ||||
| 10 | The UDP-glucuronosyltransferase 2B7 isozyme is responsible for gemfibrozil glucuronidation in the human liver. Drug Metab Dispos. 2007 Nov;35(11):2040-4. | ||||
| 11 | Comprehensive pharmacogenomic study reveals an important role of UGT1A3 in montelukast pharmacokinetics. Clin Pharmacol Ther. 2018 Jul;104(1):158-168. | ||||
| 12 | Genomic, proteomic, and metabolite characterization of gemfibrozil-degrading organism Bacillus sp. GeD10. Environ Sci Technol. 2016 Jan 19;50(2):744-55. | ||||
| 13 | Inhibiting wild-type and C299S mutant AKR1B10; a homologue of aldose reductase upregulated in cancers. Eur J Pharmacol. 2008 Apr 28;584(2-3):213-21. | ||||
| 14 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 15 | Niacin, but not gemfibrozil, selectively increases LP-AI, a cardioprotective subfraction of HDL, in patients with low HDL cholesterol. Arterioscler Thromb Vasc Biol. 2001 Nov;21(11):1783-9. doi: 10.1161/hq1001.096624. | ||||
| 16 | Expression of cytochrome P450 epoxygenases and soluble epoxide hydrolase is regulated by hypolipidemic drugs in dose-dependent manner. Toxicol Appl Pharmacol. 2018 Sep 15;355:156-163. | ||||
| 17 | Cytochrome P450 inhibition potential of new psychoactive substances of the tryptamine class. Toxicol Lett. 2016 Jan 22;241:82-94. | ||||
| 18 | Comparative effects of fibrates on drug metabolizing enzymes in human hepatocytes. Pharm Res. 2005 Jan;22(1):71-8. | ||||
| 19 | Fibrates modify the expression of key factors involved in bile-acid synthesis and biliary-lipid secretion in gallstone patients. Eur J Clin Pharmacol. 2004 Feb;59(12):855-61. doi: 10.1007/s00228-003-0704-1. Epub 2003 Dec 18. | ||||
| 20 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
| 21 | Asplund K, Wiholm BE, Lithner F "Glibenclamide-associated hypoglycaemia: a report on 57 cases." Diabetologia 24 (1983): 412-7. [PMID: 6411511] | ||||
| 22 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 23 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 24 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 25 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 26 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 27 | Ogilvie BW, Zhang D, Li W, et al. "Glucuronidation converts gemfibrozil to a potent, metabolism-dependent inhibitor of CYP2C8: implications for drug-drug interactions." Drug Metab Dispos 34 (2006): 191-7. [PMID: 16299161] | ||||
| 28 | Product Information. Vosevi (sofosbuvir/velpatasvir/voxilaprevir). Gilead Sciences, Foster City, CA. | ||||
| 29 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 30 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 31 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 32 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 33 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 34 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 35 | Product Information. Xtandi (enzalutamide). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 36 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 37 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
