Details of the Drug
General Information of Drug (ID: DMGV8QU)
| Drug Name |
Etravirine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Intelence; DAPY deriv; Diaminopyrimidine deriv; R 165335; R165335; TMC 125; TMC125; Intelence (TN); Intelence(TM); R-165335; R165335-TMC125; TMC-125; Etravirine (JAN/USAN/INN); TMC-125/R-165335; 4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)-4-pyrimidinyl)oxy)-3,5-dimethyl-benzonitrile; 4-({6-AMINO-5-BROMO-2-[(4-CYANOPHENYL)AMINO]PYRIMIDIN-4-YL}OXY)-3,5-DIMETHYLBENZONITRILE; 4-[6-amino-5-bromo-2-(4-cyanoanilino)pyrimidin-4-yl]oxy-3,5-dimethylbenzonitrile; 65B; ETR
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
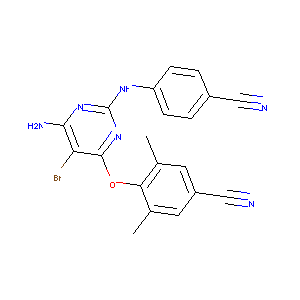 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 435.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Etravirine
Coadministration of a Drug Treating the Disease Different from Etravirine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Emerging antiviral drugs. Expert Opin Emerg Drugs. 2008 Sep;13(3):393-416. | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | ||||
| 6 | Interaction potential of etravirine with drug transporters assessed in vitro. Antimicrob Agents Chemother. 2011 Mar;55(3):1282-4. | ||||
| 7 | Pharmacokinetic interactions between etravirine and non-antiretroviral drugs. Clin Pharmacokinet. 2011 Jan;50(1):25-39. | ||||
| 8 | Biotransformation of the antiretroviral drug etravirine: metabolite identification, reaction phenotyping, and characterization of autoinduction of cytochrome P450-dependent metabolism. Drug Metab Dispos. 2012 Apr;40(4):803-14. | ||||
| 9 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 10 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 11 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 12 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 13 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 14 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 15 | Canadian Pharmacists Association. | ||||
| 16 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 17 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 18 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 19 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 20 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 21 | Product Information. Jevtana (cabazitaxel). sanofi-aventis , Bridgewater, NJ. | ||||
| 22 | Product Information. Bosulif (bosutinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 23 | Bruderer S, Aanismaa P, Homery MC, et al. "Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue-targeting dual endothelin receptor antagonist." AAPS J 14 (2012): 68-78. [PMID: 22189899] | ||||
| 24 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 25 | Patel S, Robinson R, Burk M "Hypertensive crisis associated with St. John's Wort." Am J Med 112 (2002): 507-8. [PMID: 11959071] | ||||
| 26 | Product Information. Viibryd (vilazodone). Trovis Pharmaceuticals LLC, New Haven, CT. | ||||
| 27 | Product Information. Brintellix (vortioxetine). Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 28 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 29 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 30 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 31 | Carrion C, Espinosa E, Herrero A, Garcia B "Possible vincristine-isoniazid interaction." Ann Pharmacother 29 (1995): 201. [PMID: 7756727] | ||||
| 32 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 33 | Product Information. Belsomra (suvorexant). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 34 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 35 | Product Information. Movantik (naloxegol). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 36 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 37 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 38 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 39 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 40 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 41 | Product Information. Venclexta (venetoclax). AbbVie US LLC, North Chicago, IL. | ||||
| 42 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 43 | Product Information. Imbruvica (ibrutinib). Pharmacyclics Inc, Sunnyvale, CA. | ||||
| 44 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 45 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 46 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 47 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 48 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 49 | Product Information. Jakafi (ruxolitinib). Incyte Corporation, Wilmington, DE. | ||||
| 50 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 51 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 52 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 53 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 54 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 55 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 56 | Product Information. Adempas (riociguat). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 57 | Pithavala YK, Tortorici M, Toh M, et al "Effect of rifampin on the pharmacokinetics of axitinib (AG-013736) in Japanese and Caucasian healthy volunteers." Cancer Chemother Pharmacol 65 (2010): 563-70. [PMID: 19603168] | ||||
| 58 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 59 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 60 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 61 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 62 | Product Information. Eliquis (apixaban). Bristol-Myers Squibb Canada Inc, Montreal, IN. | ||||
| 63 | Product Information. Cabometyx (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 64 | Product Information. Tradjenta (linagliptin). Boehringer Ingelheim, Ridgefield, CT. | ||||
