Details of the Drug
General Information of Drug (ID: DME2PY5)
| Drug Name |
Midodrine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alphamine; Amatine; Gutron; Hipertan; Metligene; Midamine; Midodrin; Midodrina; Midodrinum; ProAmatine; MIDODRINE HYDROCHLORIDE; Midodrine HCL; Midodrine Monohydrochloride; Midodrine hydrochloride [USAN]; A 4020 Linz; St 1085; TS 701; A-4020 Linz; Midodrina [INN-Spanish]; Midodrine (INN); Midodrine [BAN:INN]; Midodrine [INN:BAN]; Midodrine hydrochloride [USAN:JAN]; Midodrinum [INN-Latin]; Pro-Amatine; Proamatine (TN); ST-1085; St. Peter 224; Midodrine hydrochloride (JAN/USAN); Pro-Amatine (TN); DL-N1-(beta-Hydroxy-2,5-dimethoxyphenethyl)glycinamid; N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]glycinamide; N-{2-[2,5-bis(methyloxy)phenyl]-2-hydroxyethyl}glycinamide; [+/-]-1-[2,5-Dimethoxyphenyl]-2-glycinamidoethanol; Acetamide, 2-amino-N-(beta-hydroxy-2,5-dimethoxyphenethyl)-, hydrochloride; Acetamide, 2-amino-N-(2-(2,5-dimethoxyphenyl)-2-hydroxyethyl)-, monohydrochloride; (+-)-1-(2',5'-Dimethoxyphenyl)-2-glycinamidoethanol hydrochloride; (+-)-2-Amino-N-(beta-hydroxy-2,5-dimethoxyphenethyl)acetamide monohydrochloride; (+-)-Midodrine hydrochloride; (+/-)-1-(2,5-Dimethoxyphenyl)-2-glycinamidoethanol; (-)-Midodrin hydrochloride; (-)-Midodrine hydrochloride; (RS)-N'-(beta-Hydroxy-2,5-dimethoxy-phenethyl)glycinamid; 1-(2',5'-Dimethoxyphenyl)-2-glycinamidoethanol; 1-(2',5'-Dimethoxyphenyl)-2-glycinamidoethanol hydrochloride; 1-(2,5-Dimethoxyphenyl)-2-glycinamidoethanol; 2-Amino-N-(2,5-dimethoxy-beta-hydroxyphenethyl)acetamid; 2-Amino-N-(2,5-dimethoxy-beta-hydroxyphenethyl)acetamide; 2-Amino-N-(2,5-dimethoxy-beta-hydroxyphenethylacetamide; 2-Amino-N-(2-(2,5-dimethoxyphenyl)-2-hydroxyethyl)acetamide monohydrochloride; 2-Amino-N-(2-(2,5-dimethoxyphenyl)-2-hyyroxyethyl)acetamide monohydrochloride; 2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]acetamide; 2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]acetamide hydrochloride
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Vasoconstrictor Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
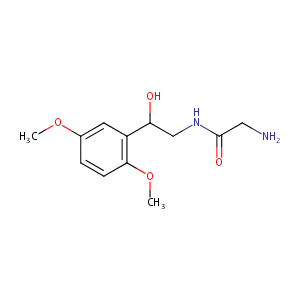 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 254.28 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Midodrine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7240). | ||||
|---|---|---|---|---|---|
| 2 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Orthostatic hypotension in patients with Parkinson's disease: pathophysiology and management. Drugs Aging. 2001;18(7):495-505. | ||||
| 6 | Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60. | ||||
| 7 | Akathisia with combined use of midodrine and promethazine. JAMA. 2006 May 3;295(17):2000-1. Letter | ||||
| 8 | Barthel W, Glusa E, Koth W "Interactions of dihydroergotamine with etilefrine in human leg veins in vitro and in situ." Int J Clin Pharmacol Ther Toxicol 25 (1987): 63-9. [PMID: 2881898] | ||||
| 9 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 10 | Product Information. Ranexa (ranolazine). Calmoseptine Inc, Huntington Beach, CA. | ||||
| 11 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 12 | Product Information. Onfi (clobazam). Lundbeck Inc, Deerfield, IL. | ||||
| 13 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 14 | Hendershot PE, Antal EJ, Welshman IR, Batts DH, Hopkins NK "Linezolid: pharmacokinetic and pharmacodynamic evaluation of coadministration with pseudoephedrine HCl, phenylpropanolamine HCl, and dextromethorpan HBr." J Clin Pharmacol 41 (2001): 563-72. [PMID: 11361053] | ||||
| 15 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 16 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 17 | Product Information. Enablex (darifenacin). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 18 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 19 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 20 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 21 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 22 | Product Information. Vizimpro (dacomitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 23 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 24 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 25 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 26 | Platts SH, Shi SJ, Meck JV "Akathisia with combined use of midodrine and promethazine." JAMA 295 (2006): 2000-1. [PMID: 16670408] | ||||
| 27 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 28 | Product Information. Meridia (sibutramine). Knoll Pharmaceutical Company, Whippany, NJ. | ||||
| 29 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 30 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
