Details of the Drug
General Information of Drug (ID: DMGR5Z3)
| Drug Name |
Flunitrazepam
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Flunibeta; Flunimerck; Fluninoc; Flunitrazepamum; Fluridrazepam; Narcozep; Primun; Rohipnol; Rohypnol; Roipnol; Betapharm Brand of Flunitrazepam; Ct Arzneimittel Brand of Flunitrazepam; Flunitrazepam Teva; Flunitrazepam neuraxpharm; Flunitrazepam ratiopharm; Flunizep von ct; Hexal Brand of Flunitrazepam; Merck dura Brand ofFlunitrazepam; Neuraxpharm Brand of Flunitrazepam; Ratiopharm Brand of Flunitrazepam; Roche Brand of Flunitrazepam; Teva Brand of Flunitrazepam; Fluni 1A Pharma; RO54200; [3H]flunitrazepam; Ct-Arzneimittel Brand of Flunitrazepam; Darkene (TN); Flunipam (TN); Flunitrazepam-Teva; Flunitrazepam-neuraxpharm; Flunitrazepam-ratiopharm; Flunitrazepamum [INN-Latin]; Hipnosedon (TN); Hypnodorm (TN); Ilman(TN); Insom (TN); Nilium (TN); Ro 5-4200; Rohypnol (TN); Rufinol (TN); Silece (TN); Vulbegal (TN); RO-5-4200; Flunitrazepam (JP15/USAN/INN); Flunitrazepam [USAN:INN:BAN:JAN]; 1,3-Dihydro-5-(o-fluorophenyl)-1-methyl-7-nitro-2H-1,4-benzodiazepin-2-one; 1-Methyl-7-nitro-5-(2-fluorophenyl)-3H-1,4-benzodiazepin-2(1H)-one; 1A Brand of Flunitrazepam; 5-(2-Fluorophenyl)-1-methyl-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 5-(2-Fluorophenyl)-1-methyl-7-nitro-3H-1,4-benzodiazepin-2(1H)-one; 5-(2-fluorophenyl)-1-methyl-7-nitro-3H-1,4-benzodiazepin-2-one; 5-(o-Fluorophenyl)-1,3-dihydro-1-methyl-7-nitro-2H-1,4-benzodiazepin-2-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
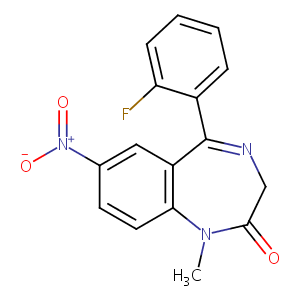 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 313.28 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
