Details of the Drug
General Information of Drug (ID: DMHM93Y)
| Drug Name |
Meprobamate
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
meprobamate; Meprospan; Meprobamat; Equanil; Miltown; Amepromat; Meprocompren; Meproban; Tranmep; Meprobam; Dapaz; Metractyl; Meprotabs; Meprosin; Meprodil; Mepiosine; Libiolan; Crestanil; Calmiren; Ayeramate; Andaksin; Anatimon; Anathylmon; Holbamate; Despasmol; Cirponyl; Biobamat; Neuramate; Metranquil; Meprocon; Mepantin; Lepetown; Ipsotian; Equilium; Equatrate; Carbaxin; Biobamate; Ansiatan; Anastress; Miltamato; Meprotil; Meprotan; Meprosan; Meproleaf; Meprindon; Mepranil; Mepavlon; Margonil; Harmonin; Dicandiol; Calmadin; Auxietil; Anxietil; Ansiowas; Amepromat; Amosene; Andaxin; Aneural; Aneurol; Aneusral; Aneuxal; Aneuxral; Ansietan; Ansil; Anural; Anzil; Apascil; Apasil; Appetrol; Arcoban; Arpon; Artolon; Ataraxine; Atraxin; Ayermate; Bamate; Brobamate; Calmax; Cirpon; Coprobate; Cypron; Cyrpon; Deprol; Diron; Diurnal; Diurnaldiverondormabrol; Diveron; Dormabrol; Ecuanil; Edenal; Enorden; Epicur; Epikur; Equinil; Equitar; Erina; Estasil; Gadexyl; Gagexyl; Hartol; Kessobamate; Klort; Larten; Lepenil; Letyl; Mendel; Mepamtin; Meposed; Mepriam; Meprin; Meprobamato; Meprobamatum; Meprodiol; Meprol; Meprosa; Meprotanum; Meproten; Meprovan; Meprovanmeprozine; Meprozine; Meptran; Meptranactylmilprem; Micrainin; Milpath; Milprem; Miltann; Miltaun; Miltuan; Miltwon; Misedant; Morbam; Multaun; Nephentine; Nervonus; Oasil; Optarket;Orlevol; Orolevol; Pancalma; Panediol; Pankalma; Pathibamate; Paxin; Pensive; Perequietil; Perequil; Perquietil; Pertranquil; Pimal; Placidon; Placitate; Prequil; Probamato; Probamyl; Probate; Procalmadiol; Procalmadol; Procalmidol; Procarbamide; Promate; Promato; Proquanil; Protran; Quaname; Quanane; Quanil; Quietidon; Quivet; Rastenil; Reostral; Restenil; Restenyl; Restinal; Restinil; Robamate;Sadanyl; Scolazil; Sedabamate; Sedanil; Sedanyl; Sedazil; Sedoquil; Sedoselecta; Selene; Seril; Setran; Shalvaton; Sowell; Spantran; Stensolo; Tamate; Tensol; Tensonal; Trancot; Trankvilan; Tranlisant; Tranquilan; Tranquilate; Tranquilax; Tranquiline; Tranquilsan; Tranquinol; Trelmar; Urbil; Urbilat; Vistabamate; Wardamate; Wyseals; Zirpon; Component of Appetrol; Component of Bamadex; Component of Equalysen; Component of Milpath; Component of Milprem; Component of Miltrate; Equanil suspension; Meprobamat [German]; Meprobamate and Aspirin Tablets; Meprobamato [Italian]; Meprobamic acid; Meprocon CMC; Solevione anastress; Bamd 400; Bamo 400; Canquil 400; Miltown 600; PMB 200; PMB 400; Apo-Meprobamate; Appetrol-Sr; Canquil-400; Component of PMB-400; Equanil (TN); Equazine-M; Fas-Cile; Fas-Cile 200; Kesso-Bamate; Mar-Bate; Meprin (VAN); Mepro-Aspirin; Mepro-analgesic; Meprobamato [INN-Spanish]; Meprobamatum [INN-Latin]; Meprospan (TN); Meprospan-200; Meprospan-400; Milprem-200; Milprem-400; Miltown (TN); My-trans; Neo-Tran; PMB-200; PMB-400; Pan-tranquil; Q-Gesic; SK-Bamate; Tranquiline (Intra); Vio-Bamate; Cap-O-Tran; Carbamic acid 2-methyl-2-propyltrimethylene ester; Meprobamate (JAN/USP/INN); Meprobamate [USAN:INN:BAN:JAN]; Carbamic acid, 2-methyl-2-propyltrimethylene ester; [2-(carbamoyloxymethyl)-2-methylpentyl] carbamate; {2-[(carbamoyloxy)methyl]-2-methylpentyl} carbamate; 1,3-Propanediol, 2-methyl-2-propyl-, 1,3-dicarbamate; 1,3-Propanediol, 2-methyl-2-propyl-, dicarbamate; 2,2-Di(carbamoyloxymethyl)pentane; 2-Methyl-2-n-propyl-1,3-propanediol dicarbamate; 2-Methyl-2-propyl-1,3-propanediol dicarbamate; 2-Methyl-2-propylpropane-1,3-diol dicarbamate; 2-Methyl-2-propyltrimethylene carbamate; 2-Metil-2-n-propil-1,3-propanediol dicarbamato; 2-Metil-2-n-propil-1,3-propanediol dicarbamato [Spanish]; 2-[(carbamoyloxy)methyl]-2-methylpentyl carbamate; 3P Bamate; KAI-1455
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antianxiety Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
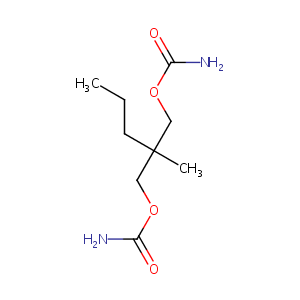 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 218.25 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.7 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Meprobamate (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Meprobamate FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7225). | ||||
| 3 | The fight against drug-resistant malaria: novel plasmodial targets and antimalarial drugs. Curr Med Chem. 2008;15(2):161-71. | ||||
| 4 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026294) | ||||
| 5 | BDDCS applied to over 900 drugs | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. | ||||
| 9 | Protein kinase C, an elusive therapeutic target . Nat Rev Drug Discov. 2012 December; 11(12): 937-957. | ||||
| 10 | Mechanisms of circadian rhythmicity of carbon tetrachloride hepatotoxicity. J Pharmacol Exp Ther. 2002 Jan;300(1):273-81. | ||||
| 11 | Solid-phase synthesis of drug glucuronides by immobilized glucuronosyltransferase. J Med Chem. 1976 May;19(5):679-83. | ||||
| 12 | Association between blood carisoprodol:meprobamate concentration ratios and CYP2C19 genotype in carisoprodol-drugged drivers: decreased metabolic capacity in heterozygous CYP2C19*1/CYP2C19*2 subjects? Pharmacogenetics. 2003 Jul;13(7):383-8. | ||||
| 13 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 14 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 15 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 16 | Gill SS, Wright EM, Reilly CS "Pharmacokinetic interaction of propofol and fentanyl: single bolus injection study." Br J Anaesth 65 (1990): 760-5. [PMID: 2265045] | ||||
| 17 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 18 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 19 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 20 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 21 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 22 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 23 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 24 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
