Details of the Drug
General Information of Drug (ID: DMPM4SK)
| Drug Name |
Methamphetamine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
METHAMPHETAMINE; Metamfetamine; d-Deoxyephedrine; d-Desoxyephedrine; d-Methamphetamine; d-N-Methylamphetamine; Metamphetamine; L-Methamphetamine; d-Methylamphetamine; Methylamphetamine; d-Phenylisopropylmethylamine; N-Methylamphetamine; (S)-Methamphetamine; Norodin; (+)-Methylamphetamine; d-(S)-Methamphetamine; D-1-Phenyl-2-methylaminopropane; Metamfetaminum; Desyphed; (+)-N-Methylamphetamine; (S)-Methylamphetamine; (+)-(S)-Deoxyephedrine; Methyl-beta-phenylisopropylamine; 1-Phenyl-2-methylaminopropane; (S)-(+)-Deoxyephedrine; Crank; Desohyephedrine; Metamfetamina; Meth; Methamphetaminum; Speed; Stimulex; Crank [Street Name]; Crystal Meth; Crystal Meth [Street Name]; Desyphed hydrochloride; ICE [Street Name]; Metamfetamine [INN];Metamfetaminum [Latin]; Metanfetamina [Spanish]; Methamphetaminum [JP]; Speed [Street Name]; D-Deoxyephedrine; D-Desoxyephedrine; D-Methamphetamine; D-Methylamphetamine; D-Phenylisopropylmethylamine;Desoxyn (TN); Metamfetamina [INN-Spanish]; Metamfetamine (INN); Metamfetamine-m; Metamfetaminum [INN-Latin]; Metanfetamina [INN-Spanish]; Meth (Street Name); Methamphetaminum [INN-Latin]; D-N-Methylamphetamine; D-(S)-Methamphetamine; D-N,alpha-dimethylphenethylamine; Methamphetamine, its salts, isomers, and salts of its isomers; N-Methyl-beta-phenylisopropylamin; N-Methyl-beta-phenylisopropylamin [German]; N-Methyl-beta-phenylisopropylamine; D-1-Phenyl-2-methylaminopropan; D-1-Phenyl-2-methylaminopropan [German]; S-(+)-Methamphetamine; D-N,alpha-Dimethylphenethylamine; N-Methyl-1-phenyl-2-propanamine; Benzeneethanamine, N,alpha-dimethyl-, (S)-(9CI); Benzeneethanamine, N,alpha-dimethyl-, (alphaS)-(9CI); Phenethylamine, N,alpha-dimethyl-, (S)-(+)-(8CI); (+)-(S)-N-alpha-Dimethylphenethylamine; (+)-2-(N-Methylamino)-1-phenylpropane; (+)-N,alpha-Dimethyl-beta-phenylethylamine; (+)-N,alpha-Dimethylphenethylamine; (+)-methamphetamine; (2S)-N-methyl-1-phenylpropan-2-amine; (S)-(+)-Methamphetamine; (S)-(+)-N,alpha,dimethylphenethylamine; (S)-N,alpha-Dimethylbenzeneethanamine; (S)-N,alpha-Dimethylbenzeneethanoamine; (alphaS)-N,alpha-dimethylbenzeneethanamine; 1-Phenyl-2-methylamino-propan; 1-Phenyl-2-methylamino-propan [German]; 2S-(+)-Methamphetamine; Methamfetamine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Central Nervous System Stimulants
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
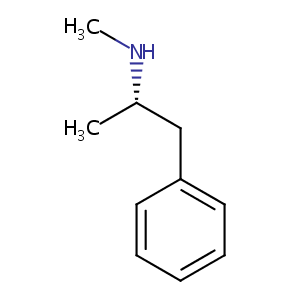 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 149.23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Anxiety | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Methamphetamine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Methamphetamine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4803). | ||||
| 3 | Pharmacotherapy for obesity. Drugs. 2005;65(10):1391-418. | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | BDDCS applied to over 900 drugs | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013 Sep;38(10):1864-70. doi: 10.1038/npp.2013.94. Epub 2013 Apr 12. | ||||
| 9 | Mirtazapine treatment after conditioning with methamphetamine alters subsequent expression of place preference. Drug Alcohol Depend. 2009 Jan 1;99(1-3):231-9. | ||||
| 10 | Synthesis and Discovery of Arylpiperidinylquinazolines: New Inhibitors of the Vesicular Monoamine Transporter. J Med Chem. 2018 Oct 25;61(20):9121-9131. | ||||
| 11 | Cytochrome P450 2D6.1 and cytochrome P450 2D6.10 differ in catalytic activity for multiple substrates. Pharmacogenetics. 2001 Aug;11(6):477-87. | ||||
| 12 | Inhibition of PLC1 signaling pathway regulates methamphetamine self-administration and neurotoxicity in rats. Food Chem Toxicol. 2021 Mar;149:111970. doi: 10.1016/j.fct.2021.111970. Epub 2021 Jan 7. | ||||
| 13 | Methamphetamine alters the normal progression by inducing cell cycle arrest in astrocytes. PLoS One. 2014 Oct 7;9(10):e109603. | ||||
| 14 | Genome-wide association for methamphetamine dependence: convergent results from 2 samples. Arch Gen Psychiatry. 2008 Mar;65(3):345-55. doi: 10.1001/archpsyc.65.3.345. | ||||
| 15 | Serotonin 1A receptor gene is associated with Japanese methamphetamine-induced psychosis patients. Neuropharmacology. 2010 Feb;58(2):452-6. doi: 10.1016/j.neuropharm.2009.09.006. Epub 2009 Sep 10. | ||||
| 16 | Serotonin 6 receptor gene is associated with methamphetamine-induced psychosis in a Japanese population. Drug Alcohol Depend. 2011 Jan 1;113(1):1-7. doi: 10.1016/j.drugalcdep.2010.06.021. Epub 2010 Aug 11. | ||||
| 17 | Anggard E, Jonsson LE, Hogmark AL, Gunne LM "Amphetamine metabolism in amphetamine psychosis." Clin Pharmacol Ther 14 (1973): 870-80. [PMID: 4729903] | ||||
| 18 | Boakes AJ, Laurence DR, Teoh PC, Barar FS, Benedikter LT, Pritchard BN "Interactions between sympathomimetic amines and antidepressant agents in man." Br Med J 1 (1973): 311-5. [PMID: 4685619] | ||||
| 19 | Product Information. Ambien (zolpidem). sanofi-aventis, Bridgewater, NJ. | ||||
| 20 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 21 | Product Information. Spravato (esketamine). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 22 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 23 | Bailey DG, Dresser GK "Natural products and adverse drug interactions." Can Med Assoc J 170 (2004): 1531-2. [PMID: 15136542] | ||||
| 24 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 25 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 26 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 27 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 28 | Product Information. Vizimpro (dacomitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 29 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 30 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 31 | Hunter KR, Boakes AJ, Laurence DR, Stern GM "Monoamine oxidase inhibitors and L-dopa." Br Med J 3 (1970): 388. [PMID: 5451592] | ||||
| 32 | Bailey DG, Arnold JMO, Spence JD "Grapefruit juice and drugs - how significant is the interaction." Clin Pharmacokinet 26 (1994): 91-8. [PMID: 8162660] | ||||
| 33 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 35 | Cusson JR, Goldenberg E, Larochelle P "Effect of a novel monoamine-oxidase inhibitor, moclobemide on the sensitivity to intravenous tyramine and norepinephrine in humans." J Clin Pharmacol 31 (1991): 462-7. [PMID: 2050833] | ||||
