Details of the Drug
General Information of Drug (ID: DMU8ANS)
| Drug Name |
Ximelegatran
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cyclohexanecarboxamide, 4-[(1R)-1-aminoethyl]-N-4-pyridinyl-, trans-; y-27632; 146986-50-7; Y-27632 dihydrochloride; Y27632; UNII-0X370ROP6H; Y 27632; cyclohexanecarboxamide, 4-[(1R)-1-aminoethyl]-N-4-pyridinyl-, trans-; 0X370ROP6H; CHEBI:75393; 4-[(1R)-1-aminoethyl]-N-(pyridin-4-yl)cyclohexane-1-carboxamide; (R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXANECARBOXAMIDE; 4-[(1R)-1-aminoethyl]-N-(4-pyridyl)cyclohexanecarboxamide; 4-[(1R)-1-aminoethyl]-N-pyridin-4-ylcyclohexane-1-carboxamide; (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide; Y-27632
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
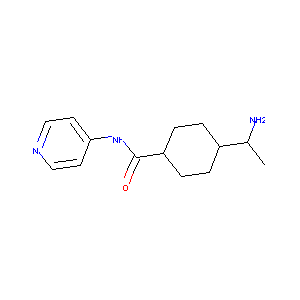 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 247.34 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
